Europe Anti Nuclear Antibody Test Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
| 2024 –2031 | |
| USD 584.64 Million | |
| USD 1,534.19 Million | |
|
|
|
|
유럽 항핵항체 검사 시장, 제품별(시약 및 검사 키트, 시스템, 소프트웨어 및 서비스), 질병(류마티스 관절염, 전신성 홍반 루푸스, 쇼그렌 증후군, 경화증, 기타 질병), 기술(ELISA, 면역형광 검사 및 다중 검사), 최종 사용자(병원, 임상 실험실, 의사 사무실 실험실 및 기타 최종 사용자), 국가(독일, 이탈리아, 영국, 프랑스, 스페인, 네덜란드, 벨기에, 스위스, 터키, 러시아, 헝가리, 리투아니아, 오스트리아, 아일랜드, 노르웨이, 폴란드 및 유럽의 나머지 지역) 산업 동향 및 2029년까지의 예측
 항핵항체 검사 시장의 시장 분석 및 통찰력
항핵항체 검사 시장의 시장 분석 및 통찰력
Data Bridge Market Research는 항핵 항체 검사가 2022-2029년 예측 기간 동안 약 12.79%의 CAGR을 보일 것이라고 분석합니다. 전 세계적으로 만성 및 자가면역 질환의 유병률 증가, 최고의 백신을 받아들이려는 지속적인 시도, 백신에 대한 임상 시험 수 증가, 의료 인프라 개발에 대한 지출 증가가 항핵 항체 검사 시장 성장에 기여하는 주요 요인입니다.
형광 항핵 항체(FANA) 및 항핵 항체 검사라고도 알려진 항핵 항체 검사는 인간의 혈청에서 발견되는 자가항체를 감지하는 데 도움이 되는 항체 검사 입니다 . 항핵 항체는 개인의 면역 체계가 질병과 싸우기 위해 자기와 이물질을 구별하지 못할 때 생성됩니다.
전 세계적으로 시약 대여 계약이 널리 퍼지는 것은 시장 성장을 촉진하는 주요 요인입니다. 의료 인프라 개발에 대한 지출 증가, 노령 인구 증가, 첨단 기술 개발 및 신제품 출시도 시장 성장을 촉진하는 다른 요인입니다. 충족되지 않은 의료적 요구가 증가하여 의료 서비스 수요가 증가하고, 의료 보험에 가입한 개인 수가 급증하고, 개인의 가처분 소득이 증가하는 것은 수익성 있는 시장 성장 기회를 창출하는 다른 간접적인 요인입니다.
그러나 개발도상국과 저개발 경제권에서 불리한 환불 시나리오는 시장 성장에 큰 도전이 될 것입니다. 의료 기기 승인에 대한 엄격한 규정은 시장 성장률에 더욱 도전이 될 것입니다. 고비용 장비를 구매하기 위해 높은 자본이 필요하면 시장 성장률이 더욱 탈선될 것입니다.
이 항핵 항체 검사 시장 보고서는 최근의 새로운 개발, 무역 규정, 수입 수출 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 항핵 항체 검사 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요 . 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
글로벌 항핵항체 검사 시장 범위 및 시장 규모
항핵 항체 검사 시장은 제품, 질병, 기술 및 최종 사용자를 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 의사 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
- 제품을 기준으로 항핵 항체 검사 시장은 시약 및 분석 키트, 시스템, 소프트웨어 및 서비스로 구분됩니다.
- 질병을 기준으로 항핵 항체 검사 시장은 류마티스 관절염, 전신성 홍반 루푸스, 쇼그렌 증후군, 경피증 및 기타 질병으로 구분됩니다.
- 기술에 기초하여 항핵항체 검사 시장은 ELISA, 면역형광 검사, 다중 검사로 구분됩니다.
- 최종 사용자를 기준으로 항핵 항체 검사 시장은 병원 , 임상 실험실, 의사 사무실 실험실 및 기타 최종 사용자로 구분됩니다.
항핵항체검사 시장 국가 수준 분석
항핵 항체 검사 시장이 분석되고, 위에 언급된 대로 국가, 제품, 질병, 기술 및 최종 사용자별로 시장 규모에 대한 통찰력과 추세가 제공됩니다.
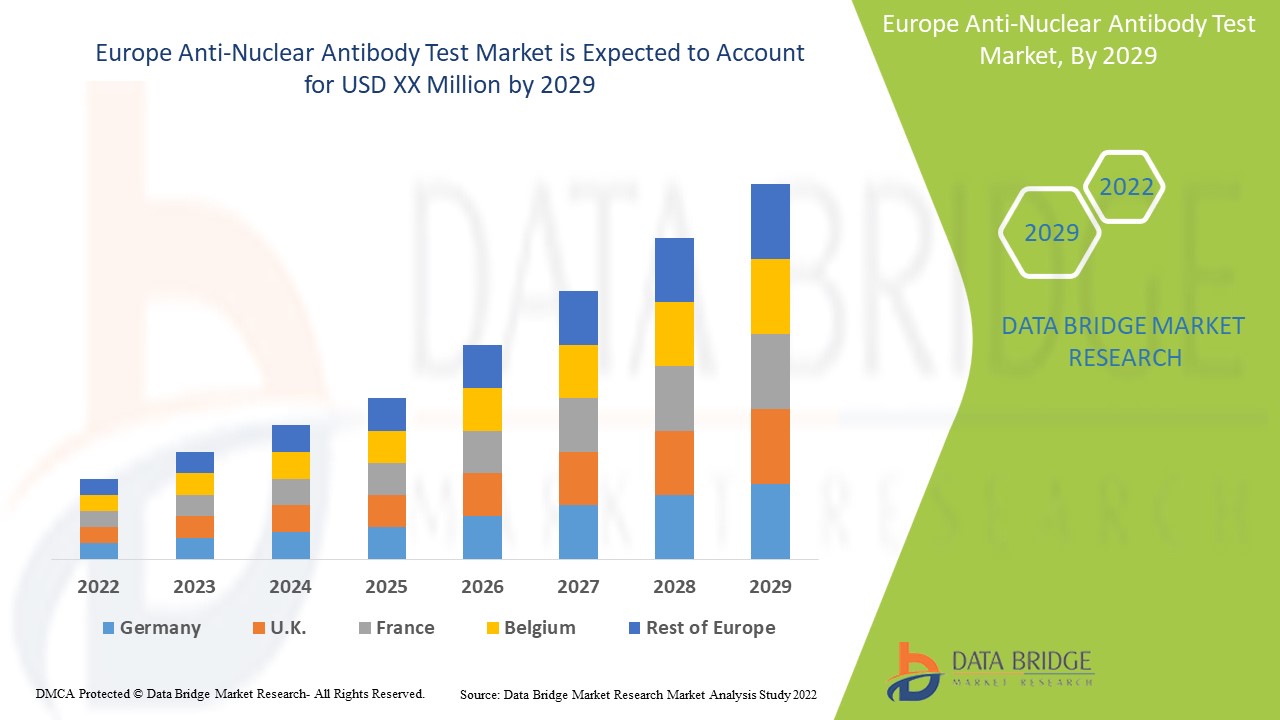
항핵 항체 검사 시장 보고서에서 다루는 국가는 독일, 이탈리아, 영국, 프랑스, 스페인, 네덜란드, 벨기에, 스위스, 터키, 러시아, 헝가리, 리투아니아, 오스트리아, 아일랜드, 노르웨이, 폴란드 및 기타 유럽 국가입니다.
유럽의 독일은 우수한 의료 인프라, 높은 환자 인지도, 첨단 검사의 존재 덕분에 항핵 항체 검사 시장을 장악하고 있습니다 .
항핵 항체 검사 시장 보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 추세에 영향을 미치는 규제 변화를 제공합니다. 소비량, 생산 현장 및 양, 수입 수출 분석, 가격 추세 분석, 원자재 비용, 하류 및 상류 가치 사슬 분석과 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
환자 역학 분석
항핵 항체 검사 시장은 또한 환자 분석, 예후 및 치료에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률, 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 항핵 항체 검사 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 항핵 항체 검사 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
경쟁 환경 및 항핵항체 검사 시장 점유율 분석
항핵 항체 검사 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우세입니다. 위에 제공된 데이터 포인트는 항핵 항체 검사 시장과 관련된 회사의 초점에만 관련이 있습니다.
항핵 항체 검사 시장 보고서에서 활동하는 주요 기업으로는 Thermo Fisher Scientific, Inc., Abbott, Bio-Rad Laboratories, Inc., Trinity Biotech Ireland, Erba Diagnostics, Antibodies Incorporated, PerkinElmer, Inc., Immuno Concepts, Inova Diagnostics, Inc., ZEUS Scientific, Inc. 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTRE’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN THE ASIA-PACIFIC REGION
5.2 NORTH AMERICA REGULATORY SCENARIO
5.3 EUROPE REGULATORY SCENARIO
5.4 REGULATORY SUBMISSIONS
5.5 KEY INTERNATIONAL AUTHORITIES
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE OF AUTOIMMUNE DISEASES ACROSS THE GLOBE
6.1.2 RISE IN AGING POPULATION
6.1.3 GROWING RESEARCH EFFORTS FOCUSED ON AUTOIMMUNE DISEASES
6.1.4 EXPANSION OF DIAGNOSTIC CENTERS AND LABORATORIES
6.2 RESTRAINTS
6.2.1 PROBLEM OF OBTAINING CONFIRMATORY RESULTS IN ANTI-NUCLEAR ANTIBODY TEST
6.2.2 LACK OF STANDARDIZATION FOR TESTING PROTOCOLS
6.3 OPPORTUNITIES
6.3.1 INTEGRATION OF DIGITAL HEALTH SOLUTIONS
6.3.2 IMPROVEMENT IN HEALTHCARE INFRASTRUCTURE
6.3.3 INCREASE IN EUROPE HEALTH INITIATIVES
6.4 CHALLENGES
6.4.1 HIGH COST OF TESTS AND EQUIPMENT
6.4.2 COMPETITION FROM ALTERNATIVE DIAGNOSTIC METHODS
7 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE
7.1 OVERVIEW
7.2 EXTRACTABLE NUCLEAR ANTIGENS (ENA)
7.2.1 ANTI-RO/SS-A AND ANTI-LA/SS-B
7.2.2 ANTI-SCL-70/ANTI-TOPOISOMERASE I
7.2.3 ANTI-NRNP/ANTI-U1-RNP
7.2.4 ANTI-SM
7.2.5 ANTI-JO-1
7.3 ANTI-DSDNA & HISTONES
7.4 ANTI-DFS70 ANTIBODIES
7.5 ANTI-PM-SCL
7.6 ANTI-CENTROMERE ANTIBODIES
7.7 ANTI-SP100
7.8 OTHERS
8 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 INSTRUMENTS
8.2.1 ANALYZERS
8.2.2 AUTOMATIC ANALYZERS
8.2.3 SEMI AUTOMATIC ANALYZERS
8.3 CONSUMABLES AND REAGENTS
8.3.1 REAGENTS
8.3.1.1 Reactive Reagents
8.3.1.2 Non Reactive Reagents
8.3.1.2.1 PBS Buffer Powder
8.3.1.2.2 Semi-Permeating Mounting Medium
8.3.1.2.3 Solutions
8.3.1.2.4 Others
8.3.2 ACCESSORIES
8.4 SERVICES
9 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE
9.1 OVERVIEW
9.2 ELISA
9.2.1 GENERIC ASSAY TECHNIQUE
9.2.2 ANTIGEN SPECIFIC ASSAY TECHNIQUE
9.3 INDIRECT IMMUNOFLUORESCENCE (IIF)
9.3.1 HEP-2 SUBSTRATE
9.3.2 CRITHIDIA LUCILIAE SUBSTRATE
9.4 BLOTTING TEST
9.4.1 DOT BLOT
9.4.2 WESTERN BLOT
9.5 ANTIGEN MICROARRAY
9.6 GEL BASED TECHNIQUES
9.6.1 COUNTER CURRENT IMMUNOELECTROPHORESIS (CIE)
9.6.2 DOUBLE IMMUNODIFFUSION (DID)
9.7 MULTIPLEX ASSAY
9.8 FLOW CYTOMETRY
9.9 PASSIVE HAEMAGGLUTINATION (PHA)
9.1 OTHERS
10 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 AUTOIMMUNE DISEASES
10.2.1 RHEUMATOID ARITHRITIS
10.2.2 SYSTEMIC LUPUS ERYTHEMATOSUS
10.2.3 SJOGREN SYNDROME
10.2.4 SCLERODERMA
10.2.5 POLYMYOSITIS
10.2.6 THYROIDITIS
10.2.7 MIXED CONNECTIVE TISSUE DISEASE (MCTD)
10.2.8 AUTOIMMUNE HEPATITIS
10.2.9 LYMPHOMAS
10.2.10 OTHERS
10.3 INFECTIOUS DISEASES
10.3.1 HEPATITIS C
10.3.2 HIV
10.3.3 EB VIRUS
10.3.4 PARVOVIRUS
10.3.5 OTHERS
11 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 LABORATORIES
11.4 DIAGNOSTIC CENTERS
11.5 RESEARCH INSTITUTES
11.6 OTHERS
12 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
12.4 THIRD PARTY DISTRIBUTOR
12.5 OTHERS
13 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 U.K.
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 TURKEY
13.1.8 BELGIUM
13.1.9 NETHERLAND
13.1.10 SWITZERLAND
13.1.11 REST OF EUROPE
14 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ABBOTT
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT/ SERVICE PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 THERMO FISHER SCIENTIFIC INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
1.1.4 PRODUCT PORTFOLIO 258
16.2.4 RECENT DEVELOPMENT
16.3 INOVA DIAGNOSTICS
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT UPDATES
16.4 EUROIMMUN MEDIZINISCHE LABORDIAGNOSTIKA AG
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 REVVITY INC.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT UPDATES
16.6 BIO-RAD LABORATORIES, INC.
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AVIVA SYSTEMS BIOLOGY CORPORATION
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 A. MENARINI DIAGNOSTICS S.R.L
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 AESKU.GROUP GMBH & CO. KG
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 ANTIBODIES INCORPORATED
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 ABNOVA CORPORATION
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 BIORBYT LTD
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 CUSABIO TECHNOLOGY LLC
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 DEMEDITEC DIAGNOSTIC GMBH
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
16.15 GENO TECHNOLOGY INC
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
16.16 IMMUNO CONCEPTS
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 LIFESPAN BIOSCIENCES, INC
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT UPDATES
16.18 MYBIOSOURCE.COM
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT UPDATES
16.19 ORIGENE TECHNOLOGIES, INC.
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 TRINITY BIOTECH
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENTS
16.21 WUHAN FINE BIOTECH CO., LTD
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT UPDATES
16.22 ZEUS SCIENTIFIC, INC.
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 2 EUROPE EXTRACTABLE NUCLEAR ANTIGENS (ENA) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 3 EUROPE EXTRACTABLE NUCLEAR ANTIGENS (ENA) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 4 EUROPE ANTI-DSDNA & HISTONES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 5 EUROPE ANTI-DFS70 ANTIBODIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 6 EUROPE ANTI-PM-SCL IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 7 EUROPE ANTI-CENTROMERE ANTIBODIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 8 EUROPE ANTI-SP100 IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 9 EUROPE OTHERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 10 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 11 EUROPE INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 12 EUROPE INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 13 EUROPE ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 14 EUROPE CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 15 EUROPE CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 16 EUROPE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 EUROPE REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 18 EUROPE NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 19 EUROPE ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 20 EUROPE SERVICES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 21 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 22 EUROPE ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 23 EUROPE ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 24 EUROPE INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 25 EUROPE INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 26 EUROPE BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 27 EUROPE BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 28 EUROPE ANTIGEN MICROARRAY IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 29 EUROPE GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 EUROPE GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 EUROPE MULTIPLEX ASSAY IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 32 EUROPE FLOW CYTOMETRY IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 EUROPE PASSIVE HAEMAGGLUTINATION (PHA) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 EUROPE OTHERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 36 EUROPE AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 EUROPE AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 38 EUROPE INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 39 EUROPE INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 40 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 41 EUROPE HOSPITALS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 42 EUROPE LABORATORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 EUROPE DIAGNOSTIC CENTERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 44 EUROPE RESEARCH INSTITUTES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 EUROPE OTHERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 47 EUROPE DIRECT TENDER IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 48 EUROPE RETAIL SALES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 49 EUROPE THIRD PARTY DISTRIBUTOR IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 50 EUROPE OTHERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 51 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 52 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 53 EUROPE EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 55 EUROPE INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 EUROPE ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 EUROPE CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 EUROPE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 59 EUROPE REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 60 EUROPE NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 61 EUROPE ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 63 EUROPE ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 64 EUROPE INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 EUROPE BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 66 EUROPE GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 67 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 68 EUROPE AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 EUROPE INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 71 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 72 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 73 GERMANY EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 74 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 75 GERMANY INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 GERMANY ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 GERMANY CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 78 GERMANY REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 GERMANY REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 80 GERMANY NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 81 GERMANY ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 83 GERMANY ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 GERMANY INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 GERMANY BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 GERMANY GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 87 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 88 GERMANY AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 89 GERMANY INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 91 GERMANY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 92 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 FRANCE EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 94 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 95 FRANCE INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 FRANCE ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 FRANCE CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 FRANCE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 FRANCE REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 FRANCE NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 101 FRANCE ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 102 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 103 FRANCE ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 104 FRANCE INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 105 FRANCE BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 106 FRANCE GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 107 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 108 FRANCE AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 109 FRANCE INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 110 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 111 FRANCE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 112 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 113 U.K. EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 114 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 115 U.K. INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 116 U.K. ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 117 U.K. CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 118 U.K. REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 119 U.K. REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 120 U.K. NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 121 U.K. ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 122 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 123 U.K. ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 124 U.K. INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 125 U.K. BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 126 U.K. GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 127 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 128 U.K. AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 129 U.K. AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 130 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 131 U.K. ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 132 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 133 ITALY EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 134 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 135 ITALY INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 136 ITALY ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 137 ITALY CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 138 ITALY REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 139 ITALY REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 140 ITALY NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 141 ITALY ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 142 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 143 ITALY ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 144 ITALY INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 145 ITALY BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 146 ITALY GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 147 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 148 ITALY AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 149 ITALY INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 150 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 151 ITALY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 152 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 153 SPAIN EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 154 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 155 SPAIN INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 156 SPAIN ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 157 SPAIN CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 158 SPAIN REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 159 SPAIN REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 160 SPAIN NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 161 SPAIN ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 162 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 163 SPAIN ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 164 SPAIN INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 165 SPAIN INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 166 SPAIN GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 167 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 168 SPAIN AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 169 SPAIN INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 170 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 171 SPAIN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 172 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 173 RUSSIA EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 174 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 175 RUSSIA INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 176 RUSSIA ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 177 RUSSIA CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 178 RUSSIA REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 179 RUSSIA REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 180 RUSSIA NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 181 RUSSIA ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 182 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 183 RUSSIA ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 184 RUSSIA INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 185 RUSSIA BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 186 RUSSIA GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 187 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 188 RUSSIA AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 189 RUSSIA INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 190 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 191 RUSSIA ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 192 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 193 TURKEY EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 194 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 195 TURKEY INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 196 TURKEY ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 197 TURKEY CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 198 TURKEY REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 199 TURKEY REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 200 TURKEY NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 201 TURKEY ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 202 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 203 TURKEY ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 204 TURKEY INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 205 TURKEY BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 206 TURKEY GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 207 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 208 TURKEY AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 209 TURKEY INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 210 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 211 TURKEY ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 212 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 213 BELGIUM EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 214 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 215 BELGIUM INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 216 BELGIUM ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 217 BELGIUM CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 218 BELGIUM REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 219 BELGIUM REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 220 BELGIUM NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 221 BELGIUM ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 222 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 223 BELGIUM ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 224 BELGIUM INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 225 BELGIUM BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 226 BELGIUM GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 227 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 228 BELGIUM AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 229 BELGIUM INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 230 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 231 BELGIUM ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 232 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 233 NETHERLANDS EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 234 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 235 NETHERLANDS INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 236 NETHERLANDS ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 237 NETHERLANDS CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 238 NETHERLANDS REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 239 NETHERLANDS REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 240 NETHERLANDS NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 241 NETHERLANDS ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 242 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 243 NETHERLANDS ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 244 NETHERLANDS INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 245 NETHERLANDS BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 246 NETHERLANDS GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 247 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 248 NETHERLANDS AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 249 NETHERLANDS INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 250 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 251 NETHERLANDS ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 252 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
TABLE 253 SWITZERLAND EXTRACTABLE NUCLEAR ANTIGENS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 254 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY PRODUCT, 2022-2031 (USD THOUSAND)
TABLE 255 SWITZERLAND INSTRUMENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 256 SWITZERLAND ANALYZERS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 257 SWITZERLAND CONSUMABLES AND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 258 SWITZERLAND REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 259 SWITZERLAND REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 260 SWITZERLAND NON-REACTIVE REAGENTS IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 261 SWITZERLAND ACCESSORIES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 262 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TECHNIQUE, 2022-2031 (USD THOUSAND)
TABLE 263 SWITZERLAND ELISA IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 264 SWITZERLAND INDIRECT IMMUNOFLUORESCENCE (IIF) IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 265 SWITZERLAND BLOTTING TEST IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 266 SWITZERLAND GEL BASED TECHNIQUES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 267 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY APPLICATION, 2022-2031 (USD THOUSAND)
TABLE 268 SWITZERLAND AUTOIMMUNE DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 269 SWITZERLAND INFECTIOUS DISEASES IN ANTI-NUCLEAR ANTIBODY TEST MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 270 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 271 SWITZERLAND ANTI-NUCLEAR ANTIBODY TEST MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 272 REST OF EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET, BY ANTIBODY TYPE, 2022-2031 (USD THOUSAND)
그림 목록
FIGURE 1 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: SEGMENTATION
FIGURE 2 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: DROC ANALYSIS
FIGURE 4 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: MARKET END USER COVERAGE GRID
FIGURE 11 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: SEGMENTATION
FIGURE 12 SEVEN SEGMENTS COMPRISE THE EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET INFECTION MARKET, BY ANTIBODY TYPE
FIGURE 13 EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 INCREASING PREVALENCE OF AUTOIMMUNE DISEASES ACROSS THE GLOBE IS EXPECTED TO DRIVE THE EUROPE ANTI-NUCLEAR ANTIBODY MARKET DURING THE FORECAST PERIOD OF 2024 - 2031
FIGURE 16 EXTRACTABLE NUCLEAR ANTIGENS (ENA) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET IN 2024 & 2031
FIGURE 17 MARKET DYNAMICS
FIGURE 18 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY ANTIBODY TYPE, 2023
FIGURE 19 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY ANTIBODY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY ANTIBODY TYPE, CAGR (2024-2031)
FIGURE 21 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY ANTIBODY TYPE, LIFELINE CURVE
FIGURE 22 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY PRODUCT, 2023
FIGURE 23 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY PRODUCT, 2024-2031 (USD THOUSAND)
FIGURE 24 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY PRODUCT, CAGR (2024-2031)
FIGURE 25 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 26 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY TECHNIQUE, 2023
FIGURE 27 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY TECHNIQUE, 2024-2031 (USD THOUSAND)
FIGURE 28 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY TECHNIQUE, CAGR (2024-2031)
FIGURE 29 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY TECHNIQUE, LIFELINE CURVE
FIGURE 30 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY APPLICATION, 2023
FIGURE 31 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY APPLICATION, 2024-2031 (USD THOUSAND)
FIGURE 32 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY APPLICATION, CAGR (2024-2031)
FIGURE 33 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 34 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY END USER, 2023
FIGURE 35 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 36 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY END USER, CAGR (2024-2031)
FIGURE 37 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY DISTRIBUTION CHANNEL, 2023
FIGURE 39 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY DISTRIBUTION CHANNEL, 2024-2031 (USD THOUSAND)
FIGURE 40 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY DISTRIBUTION CHANNEL, CAGR (2024-2031)
FIGURE 41 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: SNAPSHOT (2023)
FIGURE 43 EUROPE ANTI-NUCLEAR ANTIBODY TEST MARKET: COMPANY SHARE 2023 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.




