North America Dermatology Treatment Devices Market Size, Share, and Trends Analysis Report
Market Size in USD Billion
CAGR :
% 
 USD
1.80 Billion
USD
3.60 Billion
2024
2032
USD
1.80 Billion
USD
3.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.80 Billion | |
| USD 3.60 Billion | |
|
|
|
|
North America Dermatology Treatment Devices Market Segmentation, by type (Push Button Safety Lancet, Pressure Activated Safety Lancet, Side Button Safety Lancet), by application (Blood Glucose Testing, Hemoglobin Testing, Cholesterol Testing, Coagulation Testing), by end user (Hospitals & Clinics, Diagnostic Centers and Pathology Laboratories, Home Diagnostics, Others)- Industry Trends and Forecast to 2032
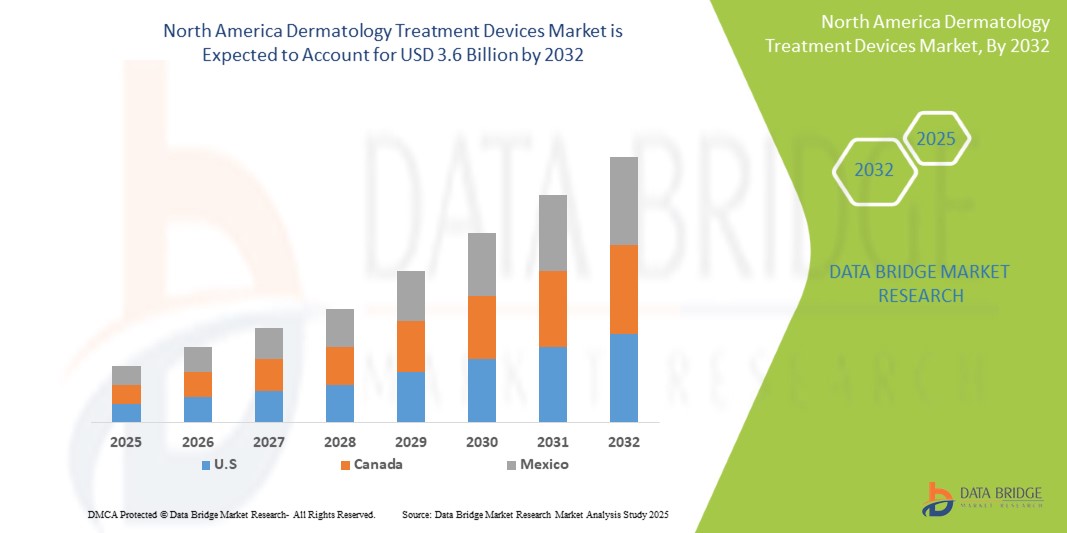
North America Dermatology Treatment Devices Market Size
- The North America Dermatology Treatment Devices Market was valued atUSD1.8 Billionin 2024and is expected to reachUSD3.6 Billionby 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 8.9%,primarily driven by the increasing prevalence of chronic diseases
- Key drivers of the North America Dermatology Treatment Devices Market include the rising prevalence of chronic diseases like diabetes, growing awareness of needle-stick injuries, and the demand for safer and more efficient blood sampling devices.
North America Dermatology Treatment Devices Market Analysis
- The increasing focus on minimizing needle-stick injuries in healthcare settings is driving the adoption of safety lancets as a safer alternative for blood sampling.
- The rise in chronic diseases, especially diabetes, is fueling the demand for blood glucose monitoring devices, including safety lancets, for at-home and clinical use.
- For instance, Innovations in lancet design, such as push-button mechanisms and pressure-activated features, are enhancing user comfort and safety, further contributing to market growth..
- Increasing awareness regarding safe and hygienic blood sampling techniques in both medical and home care settings is promoting the use of safety lancets.
- As healthcare infrastructure improves, especially in developing regions, the demand for advanced medical devices, including safety lancets, is expected to rise significantly.
Report Scope and North America Dermatology Treatment Devices Market Segmentation
|
Attributes |
North America Dermatology Treatment Devices Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Dermatology Treatment Devices Market Trends
“integration of smart technology and connectivity features”
- Enhanced Patient Monitoring and Data Collection: Smart safety lancets are equipped with features like integrated biosensors and Bluetooth connectivity, enabling real-time data transmission to digital health platforms. This facilitates continuous monitoring of blood parameters and supports proactive healthcare interventions.
- Improved Patient Compliance and Engagement: By connecting to mobile applications, these devices provide users with reminders, usage statistics, and health insights, thereby encouraging regular testing and adherence to treatment plans.
- Support for Remote Healthcare Services: The integration of smart technology aligns with the growing demand for telemedicine and home healthcare services, allowing healthcare providers to remotely monitor patients' health status and make informed decisions without the need for in-person visits.
North America Dermatology Treatment Devices Market Dynamics
Driver
“rising prevalence of chronic diseases”
- Increased Demand for Regular Blood Sampling: Chronic diseases require frequent blood tests to monitor parameters like glucose levels, cholesterol, and hemoglobin, leading to a higher need for safe and efficient blood sampling devices.
- Enhanced Patient Safety and Compliance: Safety lancets are designed to minimize the risk of needlestick injuries and cross-contamination, encouraging patients to adhere to regular testing schedules.
- Alignment with Healthcare Regulations: The adoption of safety lancets supports healthcare facilities in complying with stringent safety standards and infection control protocols, promoting their widespread use in clinical settings.
Opportunity
“development of sustainable and eco-friendly safety lancets”
- Environmental Impact Reduction: With increasing global emphasis on sustainability, manufacturers are innovating safety lancets using biodegradable materials and recyclable packaging to minimize medical waste.
- Regulatory Compliance and Market Demand: Adhering to environmental regulations and responding to consumer demand for eco-conscious products can enhance brand reputation and market share.
- Alignment with Healthcare Sustainability Goals: Integrating sustainable practices in product development aligns with the broader healthcare industry's shift towards greener solutions, potentially opening new market segments and partnerships.
Restraint/Challenge
“high cost of advanced lancet technologies”
- Increased Production Costs: Advanced safety lancets often incorporate features like adjustable penetration depths and ergonomic designs, which enhance user comfort and safety but also increase manufacturing expenses.
- Limited Accessibility in Developing Regions: The elevated costs of these advanced devices can make them less accessible in developing countries, where healthcare budgets are constrained, and cost-effective alternatives are preferred.
North America Dermatology Treatment Devices Market Scope
The market is segmented on the basis application, type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Type |
|
|
By End User |
|
North America Dermatology Treatment Devices Market Regional Analysis
“North America is the Dominant Region in the North America Dermatology Treatment Devices Market”
- High Prevalence of Chronic Diseases: The region exhibits a significant incidence of chronic conditions like diabetes, cardiovascular diseases, and cancer. For instance, in the United States, approximately 6 out of 10 adults have at least one chronic disease, necessitating regular blood sampling for management and monitoring.
- Robust Healthcare Infrastructure and Reimbursement Policies: North America boasts advanced healthcare facilities and favorable reimbursement policies, facilitating the adoption of safety lancets. In Canada, initiatives such as the Disability Tax Credit for insulin users underscore the region's commitment to supporting individuals with chronic conditions
- Technological Advancements and Market Growth: The region is witnessing continuous innovation in safety lancet technologies, including features like adjustable penetration depths and ergonomic designs. This progression, coupled with a substantial patient base and increasing healthcare expenditure, positions North America to account for a significant share of the market's growth
“Asia-Pacific is Projected to Register the Highest Growth Rate in the North America Dermatology Treatment Devices Market”
- High Prevalence of Diabetes: Over 60% of the Asian population is living with diabetes, with China and India accounting for nearly half of this number. The Western Pacific region alone has more than 138.2 million people with diabetes, a number expected to rise to 201.8 million by 2035. This surge in diabetic cases necessitates frequent blood sampling, driving demand for safety lancets.
- Government Initiatives Promoting Screening: Countries like India and China are implementing large-scale health initiatives to combat chronic diseases. For instance, India's Union Health Ministry launched a program in 2023 aiming to screen 75 million people for hypertension and diabetes by 2025. Similarly, China's 'Healthy China 2030' initiative focuses on integrated diabetes management across urban and rural areas, further increasing the need for diagnostic tools like safety lancets.
- Advancements in Healthcare Infrastructure: Rapid urbanization and improvements in healthcare services across Asia-Pacific are enhancing access to medical diagnostics. The rising incidence of infectious diseases, such as malaria, dengue, and chikungunya, which require diagnostic testing, is also contributing to the increased demand for safety lancets in the region.
North America Dermatology Treatment Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd
- Becton, Dickinson and Company (BD)
- Terumo Medical Corporation
- Bayer AG
- HTL-STREFA S.A.
- Sarstedt AG & Co. KG
- Improve Medical Technology Co. Ltd
- Ypsomed AG
- Greiner Bio-One International GmbH
- Owen Mumford Ltd
- Smiths Medical
- Nipro Corporation
- Cardinal Health, Inc.
- Arkray Inc.
- Medline Industries, Inc.
Latest Developments in North America Dermatology Treatment Devices Market
- In 2021, FUJIFILM Corporation opened NURA, a medical screening center focusing on cancer screening in Bangalore, India. This medical screening center is operated by FUJIFILM DKH LLP (FUJIFILM DKH) and Dr. Kutty’s Healthcare (DKH). FUJIFILM DKH LLP (FUJIFILM DKH) is a joint venture of FUJIFILM and Dr. Kutty’s Healthcare (DKH), which runs hospitals and screening centers in India and the Middle East.
- In 2023, Astellas Pharma announced that it has entered into an agreement with Roche Diabetes Care Japan Co., Ltd. for the development and commercialization of Roche Diabetes Care’s world-renowned Accu-Chek Guide Me blood glucose monitoring system with advanced accuracy as a combined medical product with BlueStar.
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。