北米の静脈疾患治療市場、製品タイプ別(硬化療法注射、アブレーション装置、静脈閉鎖製品、静脈ステント、薬剤など)、疾患タイプ別(深部静脈血栓症(DVT)、慢性静脈不全(CVI)、肺塞栓症、表在性血栓性静脈炎、静脈瘤など)、治療タイプ別(硬化療法、高周波アブレーション療法、レーザー治療、外来静脈切除術、静脈結紮および剥離術、血管形成術またはステント留置術、手術、圧迫療法、静脈活性薬剤、大静脈フィルターおよびその他の療法)、エンドユーザー別(病院、診療所、外来手術センターなど)、流通チャネル別(直接入札、小売販売など) - 2030年までの業界動向と予測。
北米の静脈疾患治療市場の分析と洞察
静脈疾患には、脚、腕、脳、肺、または腎臓、脾臓、肝臓などの内臓の血栓、深部静脈血栓症、慢性静脈不全、静脈瘤、静脈、静脈の潰瘍が含まれます。この疾患の治療には、薬物療法、内因性レーザー焼灼術または高周波焼灼術 (RFA)、硬化療法、および手術が含まれます。
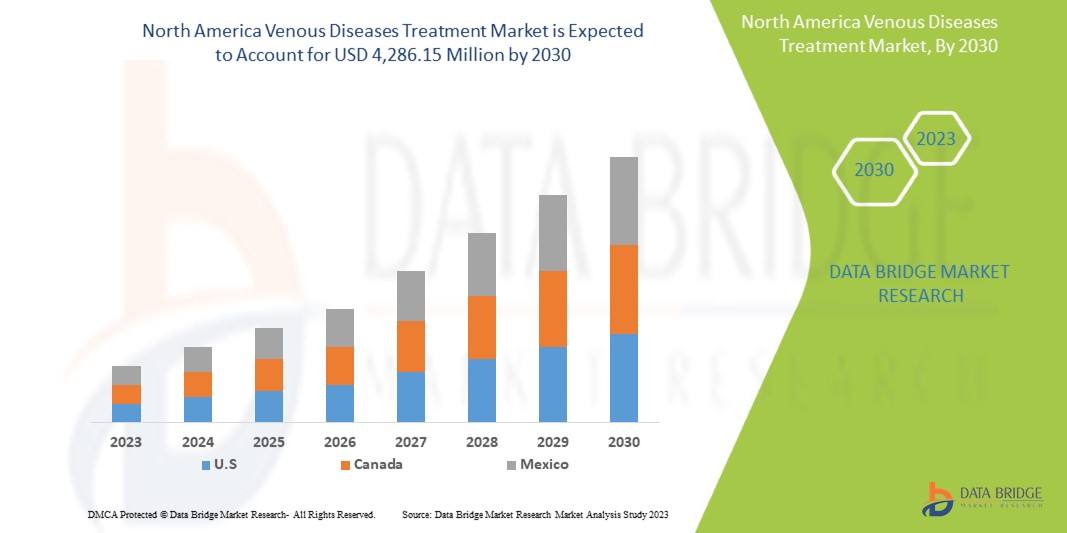
Data Bridge Market Research の分析によると、北米の静脈疾患治療市場は、予測期間中に 7.4% の CAGR で成長し、2030 年までに 42 億 8,615 万米ドルに達すると予想されています。この市場レポートでは、価格分析、特許分析、技術進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021(2020-1015にカスタマイズ可能) |
|
定量単位 |
収益は百万ドル、価格は米ドル |
|
対象セグメント |
製品タイプ別(硬化療法注射、アブレーション装置、静脈閉鎖製品、静脈ステント、薬剤など)、疾患タイプ別(深部静脈血栓症(DVT)、慢性静脈不全症(CVI)、肺塞栓症、表在性血栓性静脈炎、静脈瘤など)、治療タイプ別(硬化療法、高周波アブレーション療法、レーザー治療、外来静脈切除術、静脈結紮および剥離術、血管形成術またはステント留置術、外科手術、圧迫療法、静脈活性薬剤、大静脈フィルターおよびその他の療法)、エンドユーザー別(病院、診療所、外来手術センターなど)、流通チャネル別(直接入札、小売販売など) |
|
対象国 |
米国、カナダ、メキシコ。 |
|
対象となる市場プレーヤー |
Abbott、Imricor、Baylis Medical Company、Inc.、Theraclion、Sonablate、plusmedica.de、Boston Scientific Corporation、Olympus Corporation、Smith + Nephew、Cook、Scitech、Carl Zeiss Meditec AG、Teleflex corporate、Alma Lasers、BD、B.Braun SE、Medtronic、Stryker、Koninklijke Philips NV、Varian Medical Systems、Candela Corporation、Teromo corporation、Angiodynamics、optimed Medizinische Instrumente GmbH、Merit Medical Systems、Bolitec Laser など。 |
北米静脈疾患治療市場の定義
心臓発作や脳卒中は通常、急性の症状で、主に心臓や脳への血流を遮断する閉塞によって引き起こされます。最も一般的な原因は、心臓や脳に血液を供給する血管の内層に脂肪沈着物が蓄積することです。脳卒中は、脳の血管の出血や血栓によって引き起こされることがあります。
血管ステントの技術的進歩、低侵襲手術の需要増加、および高齢者人口の増加が北米市場を牽引しています。さらに、企業は静脈疾患治療のための最良のサービスを提供するために製品ポートフォリオを拡大しています。アブレーション装置などの医療機器は、治療目的で異常な体組織を除去または切除するための低侵襲手術で使用されます。これらのシステムは、無線周波数、エネルギー、極寒、またはレーザーによって生成された熱を使用して小さな火傷を引き起こします。製品用途の拡大のためのロボット技術の採用の増加、および患者の安全性と手順の効率を向上させるためのアブレーション装置への最先端技術の統合が、市場を牽引すると予想されます。
北米の静脈疾患治療市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 静脈疾患の発生率上昇
静脈疾患は、体内の静脈に損傷を与える病気です。血管壁が損傷すると循環器系が機能しなくなり、筋肉が弛緩すると血液が溜まり、逆流します。これにより、静脈内に異常に高い圧力が蓄積します。この蓄積により、静脈が引き締まり、ねじれ、腫れが増し、弁の機能不全が進み、血流が遅くなり、血栓ができる可能性があります。最終的に、この病気は静脈疾患と呼ばれるさまざまな病気につながる可能性があります。
加齢、肥満、高血圧、静脈疾患の家族歴などのさまざまなリスク要因により、静脈疾患の患者は世界的に増加しており、重大な社会経済的問題となっています。したがって、静脈疾患の患者数の増加により、静脈疾患治療の需要が増加し、北米の静脈疾患治療市場の推進力として機能します。
- ライフスタイルの急激な変化は肥満につながり、静脈疾患を引き起こす
喫煙、不健康な食生活、運動不足などのライフスタイルの変化は、慢性疾患、特に心臓病、脳卒中、糖尿病、肥満、メタボリックシンドロームの発症につながり、最終的には静脈疾患につながる可能性があります。
身体活動は不可欠であり、多くの病気は座りがちな生活習慣の直接的な結果です。座りがちな生活習慣は、体重増加、疲労感、原因不明の痛みや苦痛を引き起こし、中程度から重度、さらには生命を脅かす心臓血管疾患や静脈疾患などの慢性疾患を引き起こす可能性があります。
ScienceDirect の記事によると、静脈疾患は非肥満グループよりも肥満の手足で臨床的に重篤です。したがって、不健康なライフスタイルの採用の増加により、静脈疾患が増加している肥満人口が急増しています。したがって、ライフスタイルの急速な変化は肥満につながり、静脈疾患を引き起こし、静脈疾患治療の需要が増加し、北米の静脈疾患治療市場の推進力として機能します。
拘束
- 熟練した資格を持った専門家の不足
熟練した資格を持った専門家の要件は、静脈疾患治療市場にとって大きな制約となっています。ヨーロッパでは静脈疾患の症例が増加しているため、静脈疾患治療の需要は高まっていますが、医療センターに在籍する熟練した専門家の数が少ないことが市場の成長を妨げています。
慢性静脈疾患 (CVD) は、医療従事者が問題の範囲と影響を理解しておらず、一次静脈疾患と二次静脈疾患の異なる症状が十分に認識されていないため、見過ごされがちです。心血管疾患の重要性は、患者数と、より深刻な症状の社会経済的影響に関係しています。
機会
- 静脈疾患に対する意識の高まり
静脈の問題の治療には、薬物療法と生活習慣の変更の両方が利用できます。静脈は、薬物療法、運動、着圧ストッキングで改善できますが、静脈の問題によっては、静脈の健康と機能を回復するために、より徹底した治療が必要になることもあります。治療方針は、静脈疾患の種類と重症度によって決まります。過去数十年間で血管疾患の患者は劇的に増加しており、糖尿病が最も顕著なリスク要因となっています。他の健康問題とは異なり、血管疾患と血管手術は人口の約 80% に知られていません。健康意識の高まりにより、特定の病気に対する人々の態度や行動は大きく変わる可能性があります。
さまざまな社会、政府機関などによって、いくつかの啓発プログラムが実施されています。これらの取り組みにより、人々の健康に関する意識が高まり、よりよい治療と予防策のために早期診断が行われます。このため、静脈疾患に対する意識の高まりは、北米の静脈疾患治療市場の需要を伸ばす機会となることが期待されています。
チャレンジ
- 静脈疾患の治療にかかる高額な費用
米国だけで 2,500 万人以上が慢性静脈不全 (CVI) を患っており、600 万人以上が進行した静脈疾患を患っています。米国の医療制度は、CVI の発症率の高さと医療費の上昇により、財政的に大きな負担を強いられています。広範囲にわたる数多くの健康問題は、静脈循環不良が原因です。脚の痛み、浮腫、重苦しさは慢性静脈疾患 (CVD) の初期症状の一部であり、一日中続くこともあれば、夕方に顕著になることもあります。患者は、静脈瘤の有無にかかわらず起こりうる症状の初期治療や、美容上の静脈瘤除去を求めることがよくあります。CVD の 2 つの主なリスク要因は、高齢化と体重過多です。
COVID-19後の北米静脈疾患治療市場への影響
パンデミックはメーカーだけでなくユーザーにも悪影響を及ぼしました。静脈瘤の手術は緊急性が低いため、手術件数は大幅に減少しました。旅行制限により静脈瘤の治療のための手術や処置が減少したことも、メーカーの売上に影響を与えました。
メーカーは、COVID-19後の回復に向けてさまざまな戦略的決定を下しています。各社は、ペットフードのフレーバーと原料市場に関わる技術とテスト結果を改善するために、複数の研究開発活動、製品の発売、戦略的パートナーシップを行っています。
最近の動向
- 2022年7月、北米の医療技術企業であるスミス・ネフューは、創傷ケアにおける実践のばらつきを減らすための臨床サポートアプリをリリースしました。WOUND COMPASS臨床サポートアプリは、医療従事者向けの包括的なデジタルサポートツールで、創傷の評価と意思決定を支援し、実践のばらつきを減らすのに役立ちます。これにより、同社はヘルスケア顧客を引き付け、製品ポートフォリオを拡大することができました。
- 2022年4月、カールツァイスメディテックは、ソリューションプロバイダーとしての地位をさらに強化するために、外科用器具メーカー2社(Kogent Surgical、LLCおよびKatalyst Surgical、LLC)の買収を発表しました。これにより、同社は事業を拡大することができました。
北米の静脈疾患治療市場の範囲
北米の静脈疾患治療市場は、製品タイプ、疾患タイプ、治療タイプ、エンドユーザー、流通チャネルに区分されています。セグメント間の成長は、ニッチな成長分野と市場へのアプローチ戦略を分析し、コアアプリケーション領域とターゲット市場の違いを決定するのに役立ちます。
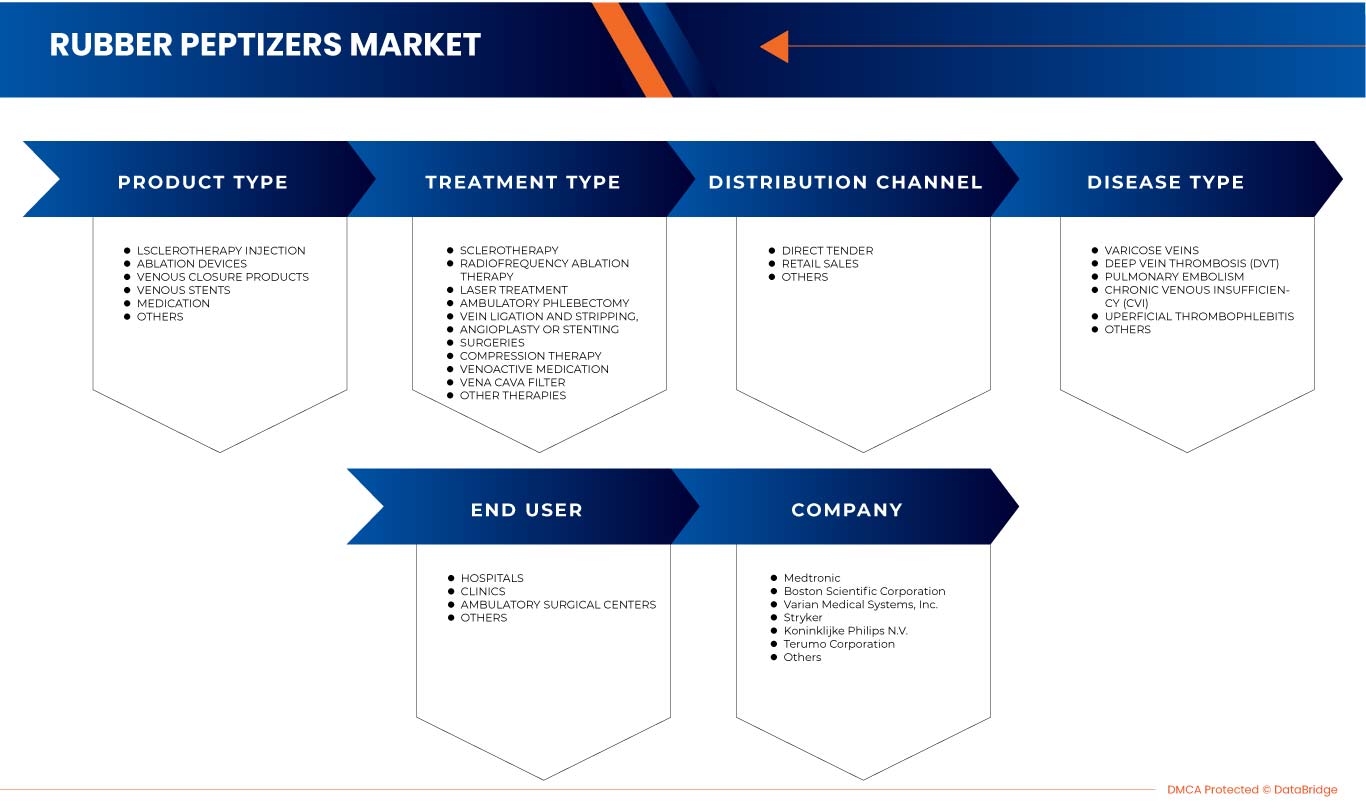
北米の静脈疾患治療市場(製品タイプ別)
- アブレーション装置
- 静脈ステント
- 静脈閉鎖製品
- 硬化療法注射
- 医薬品
- その他
製品タイプに基づいて、北米の静脈疾患治療市場は、アブレーション装置、静脈ステント、静脈閉鎖製品、硬化療法注射、医薬品、その他に分類されます。
北米の静脈疾患治療市場(疾患タイプ別)
- 深部静脈血栓症(DVT)
- 慢性静脈不全(CVI)
- 肺塞栓症
- 表在性血栓性静脈炎
- 静脈瘤
- その他
疾患の種類に基づいて、北米の静脈疾患治療市場は、深部静脈血栓症(DVT)、慢性静脈不全(CVI)、肺塞栓症、表在性血栓性静脈炎、静脈瘤、その他に分類されます。
北米の静脈疾患治療市場(治療タイプ別)
- 圧迫療法
- VENO ACTIVE メディケーション
- 手術
- 硬化療法
- 血管形成術またはステント留置術
- 静脈結紮術と静脈剥離術
- 大静脈フィルター
- 外来静脈切除術
- 高周波アブレーション療法
- レーザー治療
- その他の治療法
治療の種類に基づいて、北米の静脈疾患治療市場は、圧迫療法、静脈活性薬、手術、硬化療法、血管形成術またはステント留置、静脈結紮術および剥離術、大静脈フィルター、外来静脈切除術、高周波アブレーション療法、レーザー治療、およびその他の治療法に分類されます。
北米の静脈疾患治療市場(エンドユーザー別)
- 病院
- クリニック
- 外来手術センター
- その他
エンドユーザーに基づいて、北米の静脈疾患治療市場は、病院、診療所、外来手術センター、その他に分類されます。
北米の静脈疾患治療市場(流通チャネル別)
- 直接入札
- 小売販売
- その他
流通チャネルに基づいて、北米の静脈疾患治療市場は、直接入札、小売販売、その他に分類されます。
北米静脈疾患治療市場地域分析/洞察
北米の静脈疾患治療市場が分析され、製品タイプ、疾患タイプ、治療タイプ、エンドユーザー、流通チャネル別に市場規模情報が提供されます。
この市場レポートでカバーされている国は、米国、カナダ、メキシコです。
米国は、高い GDP を誇る最大の消費者市場に主要な市場プレーヤーが存在するため、北米で優位に立っています。米国は、静脈疾患の治療における最新の高度な技術と発明により、成長が期待されています。
レポートの国別セクションでは、市場の現在および将来の傾向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。新規販売、交換販売、国の人口統計、規制行為、輸出入関税などのデータ ポイントは、各国の市場シナリオを予測するために使用される主要な指標の一部です。また、国別データの予測分析を提供する際には、北米ブランドの存在と可用性、地元および国内ブランドとの競争が激しいか少ないために直面する課題、販売チャネルの影響も考慮されます。
競争環境と北米の静脈疾患治療市場シェア分析
北米の静脈疾患治療市場の競争状況は、競合他社ごとに詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、技術ライフライン曲線などがあります。提供されている上記のデータ ポイントは、北米の静脈疾患治療市場への会社の重点にのみ関連しています。
北米の静脈疾患治療市場で活動している主要企業には、Abbott、Imricor、Baylis Medical Company、Inc.、Theraclion、Sonablate、plusmedica.de、Boston Scientific Corporation、Olympus Corporation、Smith + Nephew、Cook、Scitech、Carl Zeiss Meditec AG、Teleflex corporate、Alma Lasers、BD、B.Braun SE、Medtronic、Stryker、Koninklijke Philips NV、Varian Medical Systems、Candela Corporation、Teromo corporation、Angiodynamics、optimed Medizinische Instrumente GmbH、Merit Medical Systems、Bolitec Laserなどがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 INSTRUMENT BASED LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING INCIDENCES OF VENOUS DISEASES
8.1.2 RAPID CHANGES IN LIFESTYLE LEAD TO OBESITY RESULTING IN VENOUS DISEASES
8.1.3 INCREASE IN THE GERIATRIC POPULATION
8.1.4 TECHNOLOGICALLY ADVANCEMENT IN THE TREATMENT OF VENOUS DISEASES
8.2 RESTRAINTS
8.2.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
8.2.2 INADEQUATE REIMBURSEMENT COVERAGE
8.3 OPPORTUNITIES
8.3.1 RISING AWARENESS TOWARDS VENOUS DISORDERS
8.3.2 NEED FOR PROPER DIAGNOSIS AND TREATMENT OF VENOUS DISEASES
8.3.3 GROWING PREFERENCE FOR MINIMALLY INVASIVE PROCEDURES
8.3.4 OCCUPATIONAL LIFESTYLE INCREASES THE NEED FOR VENOUS DISEASE TREATMENT
8.4 CHALLENGES
8.4.1 HIGH COST ASSOCIATED WITH VENOUS DISEASE TREATMENT
8.4.2 SIDE EFFECTS AND RISK ASSOCIATED WITH DIFFERENT TREATMENT MODES
9 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 SCLEROTHERAPY INJECTION
9.2.1 SCLEROTHERAPY, BY TYPE
9.2.1.1 INTRAVENOUS
9.2.1.2 INTRADERMAL
9.2.1.3 SUBCUTANEOUS
9.2.2 SUBCUTANEOUS, BY APPLICATION
9.2.2.1 MALFORMED LYMPHED VESSELS
9.2.2.2 HEMORRHOIDS
9.2.2.3 HYDROCELES
9.2.2.4 OTHERS
9.3 ABLATION DEVICES
9.3.1 THERMAL ABLATION
9.3.1.1 RADIOFREQUENCY
9.3.1.1.1 CATHETER MANIPULATION SYSTEMS
9.3.1.1.2 TEMPERATURE CONTROLLED
9.3.1.1.3 FLUID COOLED
9.3.1.2 LIGHT
9.3.1.2.1 EXCIMER LASERS
9.3.1.2.2 COLD LASERS
9.3.1.3 ULTRASOUND
9.3.1.3.1 HIGH INTENSITY FOCUSED ULTRASOUND (HIFU)
9.3.1.3.2 SHOCK WAVE LITHOTRIPSY
9.3.1.3.3 MAGNETIC RESONANCE IMAGING GUIDED FOCUSED ULTRASOUND (MRI-FUS)
9.3.1.3.4 ULTRASONIC SURGICAL SYSTEMS
9.3.1.4 RADIATION
9.3.1.4.1 STEREOTACTIC BODY RADIATION THERAPY
9.3.1.4.2 INTENSITY-MODULATED RADIATION THERAPY
9.3.1.4.3 STEREOTACTIC RADIOTHERAPY & RADIOSURGERY
9.3.1.4.4 IMAGE GUIDED RADIATION THERAPY
9.3.1.4.5 INTRAVASCULAR BRACHYTHERAPY
9.3.1.4.6 PROTON BEAM THERAPY
9.3.1.5 ELECTRICAL
9.3.1.5.1 ELECTRICAL ABLATORS
9.3.1.5.2 ELECTRONIC BRACHYTHERAPY
9.3.1.6 MICROWAVE
9.3.1.7 HYDROTHERMAL
9.3.2 NON-THERMAL ABLATION
9.3.2.1 CRYOABLATION
9.3.2.1.1 EPIDERMAL AND SUBCUTANEOUS CRYOABLATION DEVICES
9.3.2.1.2 CRYOGEN SPRAY PROBE
9.3.2.1.3 TISSUE CONTACT PROBE
9.3.2.2 HYDROMECHANICAL ABLATION
9.4 VENOUS CLOSURE PRODUCTS
9.4.1 VENOUS CLOSURE PRODUCTS, BY PROCEDURE
9.4.1.1 INTERVENTIONAL CARDIOLOGY
9.4.1.2 INTERVENTIONAL RADIOLOGY
9.4.2 VENOUS CLOSURE PRODUCTS, BY TECHNOLOGY
9.4.2.1 FEMORAL ACCESS TECHNIQUE
9.4.2.2 RADIAL ACCESS TECHNIQUE
9.5 VENOUS STENTS
9.5.1 VENOUS STENTS, BY TECHNOLOGY
9.5.1.1 WALLSTENT TECHNOLOGY
9.5.1.2 ILIAC VEIN STENT TECHNOLOGY
9.5.2 VENOUS STENTS, BY APPLICATION
9.5.2.1 LEG
9.5.2.2 CHEST
9.5.2.3 ABDOMEN
9.5.2.4 OTHERS
9.6 MEDICATION
9.7 OTHERS
10 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 VARICOSE VEINS
10.3 DEEP VEIN THROMBOSIS (DVT)
10.4 PULMONARY EMBOLISM
10.5 CHRONIC VENOUS INSUFFICIENCY (CVI)
10.6 SUPERFICIAL THROMBOPHLEBITIS
10.7 OTHERS
11 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE
11.1 OVERVIEW
11.2 SCLEROTHERAPY
11.3 RADIOFREQUENCY ABLATION THERAPY
11.4 LASER TREATMENT
11.5 AMBULATORY PHLEBECTOMY
11.6 VEIN LIGATION AND STRIPPING
11.7 ANGIOPLASTY OR STENTING
11.8 SURGERIES
11.9 COMPRESSION THERAPY
11.1 VEINACTIVE MEDICATION
11.11 VENA CAVA FILTER
11.12 OTHER THERAPIES
12 NORTH AMERICA VENOUS TREATMENT DISEASES MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
13.4 OTHERS
14 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 MEDTRONIC
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.2 BOSTON SCIENTIFIC CORPORATION
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 VARIAN MEDICAL SYSTEMS, INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 COMPANY SHARE ANALYSIS
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENT
17.4 STRYKER
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 KONINKLIJKE PHILIPS N.V.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 ABBOTT
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 ALMA LASERS
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 ANGIODYNAMICS
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENT
17.9 B.BRAUN MELSUNGEN AG
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 BAYLIS MEDICAL COMPANY, INC
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 BD
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 CANDELA MEDICAL
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.13 CARL ZEISS MEDITEC AG
17.13.1 COMPANYSNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 COOK
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENTS
17.15 IMRICOR
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 OLYMPUS CORPORATION
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOILIO
17.16.4 RECENT DEVELOPMENT
17.17 OPTIMED MEDIZINISCHE INSTRUMENTE GMBH
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENTS
17.18 PLUSMEDICA.DE
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 SCITECH
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENTS
17.2 SMITH + NEPHEW
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.21 SONABLATE
17.21.1 COMPANY SNAPSHOT
17.21.2 PRODUCT PORTFOLIO
17.21.3 RECENT DEVELOPMENT
17.22 THERACLION
17.22.1 COMPANY SNAPSHOT
17.22.2 REVENUE ANALYSIS
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENT
17.23 TELEFLEX INCORPORATED
17.23.1 COMPANY SNAPSHOT
17.23.2 REVENUE ANALYSIS
17.23.3 PRODUCT PORTFOLIO
17.23.4 RECENT DEVELOPMENTS
17.24 TERUMO CORPORATION
17.24.1 COMPANY SNAPSHOT
17.24.2 REVENUE ANALYSIS
17.24.3 PRODUCT PORTFOLIO
17.24.4 RECENT DEVELOPMENTS
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA ILIAC VEIN STENT TECHNOLOGY IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA MEDICATION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA VARICOSE VEINS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA DEEP VEIN THROMBOSIS (DVT) IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA PULMONARY EMBOLISM IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA CHRONIC VENOUS INSUFFICIENCY (CVI) IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA SUPERFICIAL THROMBOPHLEBITIS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA SCELEROTHERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA RADIOFREQUENCY ABLATION THERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA LASER TREATMENT IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA AMBULATORY PHELEBECTOMY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA VEIN LIGATION AND STRIPPING IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA ANGIOPLASTY OR STENTING IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA SURGERIES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA COMPRESSION THERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA VEINACTIVE MEDICATION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA VENA CAVA FILTER IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA OTHER THERAPIES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA HOSPITALS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CLINICS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIRECT TENDER IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA RETAIL SALES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 72 U.S. VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 U.S. SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 U.S. SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 75 U.S. ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 U.S. THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 77 U.S. RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 78 U.S. LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 U.S. ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.S. RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.S. ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.S. NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.S. CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.S. VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 85 U.S. VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 86 U.S. VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 87 U.S. VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 88 U.S. VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 89 U.S. VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 90 U.S. VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 U.S. VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 92 CANADA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 93 CANADA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 CANADA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 95 CANADA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CANADA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 CANADA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 CANADA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 CANADA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 CANADA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 CANADA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 CANADA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 CANADA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 104 CANADA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 105 CANADA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 106 CANADA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 107 CANADA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 108 CANADA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 109 CANADA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 110 CANADA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 111 MEXICO VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 112 MEXICO SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 113 MEXICO SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 114 MEXICO ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 MEXICO THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 MEXICO RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 MEXICO LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 MEXICO ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 MEXICO RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MEXICO ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 MEXICO NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 MEXICO CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 123 MEXICO VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 124 MEXICO VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 125 MEXICO VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 126 MEXICO VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 127 MEXICO VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 128 MEXICO VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 129 MEXICO VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 MEXICO VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS ABOUT VENOUS DISEASES TREATMENTS AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET FROM 2023 TO 2030
FIGURE 12 THE ABLATION DEVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET
FIGURE 14 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, 2022
FIGURE 19 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, 2022
FIGURE 23 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, 2022
FIGURE 27 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SNAPSHOT (2022)
FIGURE 35 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2022)
FIGURE 36 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。