中東およびアフリカのアダリムマブ市場
Market Size in USD Billion
CAGR :
% 
 USD
134.81 Million
USD
181.68 Million
2021
2029
USD
134.81 Million
USD
181.68 Million
2021
2029
| 2022 –2029 | |
| USD 134.81 Million | |
| USD 181.68 Million | |
|
|
|
中東およびアフリカのアダリムマブ市場、薬物クラス別(抗リウマチ薬、TNFアルファ阻害剤、その他)、適応症別(関節リウマチ、強直性脊椎炎、慢性尋常性乾癬、クローン病、潰瘍性大腸炎、乾癬性関節炎、若年性特発性関節炎、化膿性汗腺炎、非感染性中間体、その他)、タイプ別(生物学的製剤、バイオシミラー)、用量強度別(40mg/0.4mlg、80mg/0.8mlg、20mg/0.2mlg、10mg/0.1mlg、その他)、薬物タイプ別(ブランド、ジェネリック)、投与経路別(経口、非経口、その他)、年齢層別(小児、成人、高齢者)、剤形別(錠剤、注射剤、溶液、その他)、エンドユーザー(病院、専門クリニック、在宅ケア、その他)、流通チャネル(病院薬局、小売薬局、オンライン薬局、その他) - 2029年までの業界動向と予測
市場分析と規模
米国で最初に認可されたアダリムマブは、現在 60 か国以上で入手可能です。中東およびアフリカ市場は統合されており、価格面で互いに競い合っている企業はわずか数社です。大手企業のほとんどは現在、関節リウマチおよび乾癬の治療薬としてアダリムマブのバイオシミラーの開発に力を注いでいます。これは、医学的疾患の治療におけるアダリムマブのバイオシミラーの安全性と有効性をテストする臨床試験で明らかです。成人の炎症性疾患の多くは、潰瘍性大腸炎、関節リウマチ、乾癬性関節炎、強直性脊椎炎、尋常性乾癬、化膿性汗腺炎など、アダリムマブで治療されています。
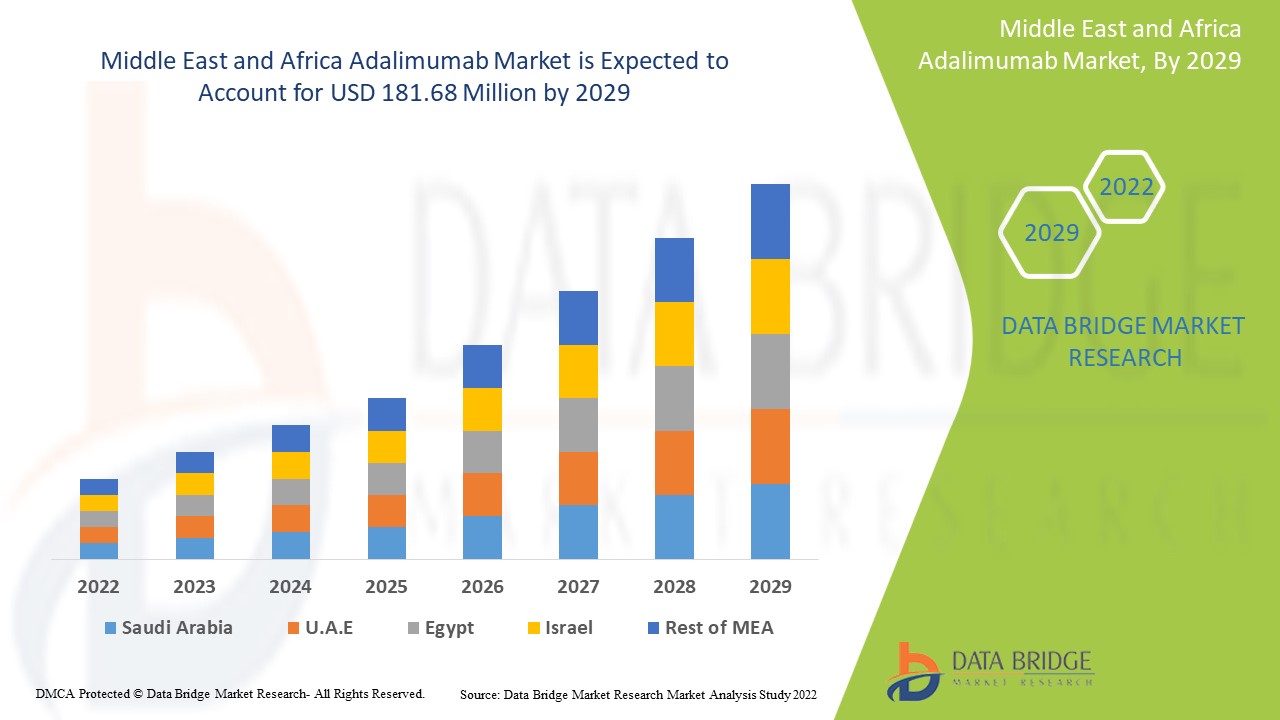
データブリッジ市場調査は、中東およびアフリカのアダリムマブ市場は2021年に1億3,481万米ドルと評価され、2022年から2029年の予測期間中に3.80%のCAGRを記録し、2029年には1億8,168万米ドルに達すると予測しています。データブリッジ市場調査チームがまとめた市場レポートには、市場価値、成長率、市場セグメント、地理的範囲、市場プレーヤー、市場シナリオなどの市場洞察に加えて、詳細な専門家分析、患者疫学、パイプライン分析、価格分析、規制枠組みも含まれています。
レポートの範囲と市場セグメンテーション
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 (2014 - 2019 にカスタマイズ可能) |
|
定量単位 |
売上高(百万米ドル)、販売数量(個数)、価格(米ドル) |
|
対象セグメント |
薬剤クラス(抗リウマチ薬、TNF アルファ阻害剤、その他)、適応症(関節リウマチ、強直性脊椎炎、慢性尋常性乾癬、クローン病、潰瘍性大腸炎、乾癬性関節炎、若年性特発性関節炎、化膿性汗腺炎、非感染性中間体、その他)、タイプ(生物学的製剤、バイオシミラー)、用量(40mg/0.4mlg、80mg/0.8mlg、20mg/0.2mlg、10mg/0.1mlg、その他)、薬剤タイプ(ブランド、ジェネリック)、投与経路(経口、非経口、その他)、年齢層(小児、成人、高齢者)、剤形(錠剤、注射剤、溶液、その他)、エンドユーザー(病院、専門クリニック、ホームケア、その他)、流通チャネル(病院薬局、小売薬局、オンライン薬局、その他) |
|
対象国 |
サウジアラビア、UAE、南アフリカ、エジプト、イスラエル、中東およびアフリカ (MEA) の残りの国々 (中東およびアフリカ (MEA) の一部)。 |
|
対象となる市場プレーヤー |
Mylan NV (米国)、AbbVie Inc. (米国)、Zydus Cadila (インド)、Pfizer Inc. (米国)、Hetero Biopharma Ltd. (インド)、Boehringer Ingelheim International GmbH. (ドイツ) |
|
市場機会 |
|
市場の定義
アダリムマブは、Humira および Exemptia というブランド名で販売されている処方薬です。関節リウマチ、乾癬性関節炎、クローン病、乾癬、および潰瘍性大腸炎はすべてアダリムマブで治療されます。アダリムマブは一般に TNF (腫瘍壊死因子アルファ) と結合します。TNF が TBF 受容体と相互作用すると、自己免疫疾患に対する炎症反応が誘発されます。TNF に結合することで、アダリムマブは炎症反応の可能性を減らします。
中東およびアフリカの アダリムマブ市場の動向
ドライバー
- 自己免疫疾患の発症率の上昇
乾癬性関節炎、尋常性乾癬、潰瘍性大腸炎、強直性脊椎炎、関節リウマチ、クローン病などの自己免疫疾患の発生率の上昇により、市場の成長率が上昇すると予想されています。アダリムマブは、痛みや腫れを軽減すると同時に、関節炎の進行を遅らせる薬です。アダリムマブは、活動性付着部炎関連関節炎、関節リウマチ、変形性関節症、多関節性若年性特発性関節炎、その他の自己免疫疾患の治療に使用されます。これに伴い、慢性疾患の蔓延が進むことで、アダリムマブ市場の需要が高まります。
- 医療インフラへの投資増加
アダリムマブ市場の成長率に影響を与えるもう一つの重要な要因は、インフラの改善に役立つ医療費の増加です。また、さまざまな政府機関が資金を増やすことで医療インフラの改善を目指しており、これが市場の動向にさらに影響を与えるでしょう。
- 皮膚疾患の発生率上昇
The incidences of skin disorders is estimated to propel the market’s growth rate during the forecast period of 2022-2029. The World Health Organization (WHO) estimates that 900 million people worldwide suffer from skin ailments at any given moment. TNF-alpha (tumor necrosis factor-alpha) is a critical participant in the inflammatory process that causes skin disorders including psoriasis. Adalimumab targets this protein in the body. Psoriasis is a skin condition that develops scaly red patches on the knees, elbows, trunk, and scalp. Psoriasis is caused by an overactive immune system response, which is suppressed by adalimumab. According to the National Psoriasis Foundation, 125 million individuals worldwide have psoriasis, accounting for 2 to 3 percent of the overall population, fueling market growth.
Furthermore, rising initiatives by public and private organizations to spread awareness and surging demand for biosimilar drugs owing to their cost-effectiveness will expand the adalimumab market. Additionally, surging number of geriatric population and rising cases of upper respiratory tract infection will result in the expansion of adalimumab market.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by an increase in the number of research and development activities. This will provide beneficial opportunities for the adalimumab market growth. Along with this, rising drug approvals and launches will further propel the market’s growth rate.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the adalimumab market growth during the forecast period.
Restraints/Challenges
- High cost as well as side effects associated with adalimumab
Adalimumab is quite expensive for persons in low and middle-income nations, costing roughly USD 2000-3000 each infusion. Furthermore, adalimumab's negative effects are expected to limit market expansion. Fever, swollen glands, night sweats, general sensation of unwell, joint and muscle pain, skin rash, easy bruising or bleeding, and others are some of the frequent adverse effects of adalimumab. Adalimumab can also cause a type of lymphoma that is fatal, as well as cancers of the liver, spleen, and bone marrow. This is most common in teenagers and young men with Crohn's disease or ulcerative colitis, which slows market growth.
On the other hand, the lack of healthcare infrastructure in developing economies and strict regulatory process linked with product approval of biosimilars will challenge the adalimumab market. Additionally, patent expiration of drugs will act as restrain and further impede the growth rate of market during the forecast period of 2022-2029.
この中東およびアフリカのアダリムマブ市場レポートは、最近の新しい開発、貿易規制、輸出入分析、生産分析、バリュー チェーンの最適化、市場シェア、国内および現地の市場プレーヤーの影響、新たな収益源の観点から見た機会の分析、市場規制の変更、戦略的市場成長分析、市場規模、カテゴリ市場の成長、アプリケーションのニッチと優位性、製品の承認、製品の発売、地理的拡張、市場における技術革新の詳細を提供します。中東およびアフリカのアダリムマブ市場の詳細については、アナリスト ブリーフについて Data Bridge Market Research にお問い合わせください。当社のチームが、情報に基づいた市場決定を行い、市場の成長を達成できるようお手伝いします。
患者疫学分析
中東およびアフリカのアダリムマブ市場では、患者分析、予後、治療法に関する詳細な市場分析も提供されます。有病率、発症率、死亡率、遵守率は、レポートで利用できるデータ変数の一部です。疫学の市場成長への直接的または間接的な影響分析は、成長期の市場を予測するためのより堅牢なコホート多変量統計モデルを作成するために分析されます。
COVID-19による中東・アフリカの アダリムマブ市場への影響
COVID-19ウイルスは2019年12月に出現して以来、 地球上のほぼすべての国に広がり、世界保健機関(WHO)は公衆衛生上の緊急事態を宣言しました。新しいコロナウイルスであるCOVID-19は、肺炎症例の原因物質として特定されました。このウイルスは世界中に急速に広がり、多数の死者を出しました。COVID-19は、2020年3月に世界保健機関(WHO)によって中東およびアフリカのパンデミックと分類され、病気の拡大を防ぐための厳格な対策が推奨されました。それ以来、パンデミックはヘルスケアセクターの拡大を遅らせ、サプライチェーンを混乱させました。さらに、多くの国の政府はCOVID-19の拡散を阻止するために全国的なロックダウンを実施しました。同様に、世界中の多くの国のヘルスケア組織は、サプライチェーン活動の継続に苦労していました。アダリムマブ市場は、サプライチェーンの遅さによって妨げられました。
最近の開発
- 2021年10月、米国食品医薬品局(FDA)は、さまざまな炎症性疾患の治療薬として初の互換性のあるバイオシミラー製品の承認を発表しました。バイオシミラーと互換性のある承認経路は、重篤な病状の患者がより多くの治療オプションを利用できるようにするために確立されました。Cyltezoは、FDAが承認した初の互換性のあるモノクローナル抗体であり、2番目の互換性のあるバイオシミラー医薬品です。
中東およびアフリカのアダリムマブ市場の範囲
中東およびアフリカのアダリムマブ市場は、薬物クラス、タイプ、適応症、投与形態、投与量、薬物タイプ、投与経路、年齢層、エンドユーザー、流通チャネルに基づいてセグメント化されています。これらのセグメントの成長は、業界のわずかな成長セグメントを分析するのに役立ち、ユーザーに貴重な市場概要と市場洞察を提供し、コア市場アプリケーションを特定するための戦略的決定を下すのに役立ちます。
薬物クラス
- 抗リウマチ薬
- TNFアルファ阻害剤
- その他
表示
- 関節リウマチ
- 強直性脊椎炎
- 慢性尋常性乾癬
- クローン病
- 潰瘍性大腸炎
- 乾癬性関節炎
- 若年性特発性関節炎
- 化膿性汗腺炎
- 非感染性中間体
- その他
タイプ
- 生物学的製剤
- バイオシミラー
用量強度
- 40mg/0.4mlg
- 80mg/0.8mlg
- 20mg/0.2mlg
- 10mg/0.1mlg
- その他
薬剤の種類
- ブランド
- ジェネリック
投与経路
- オーラル
- 非経口
- その他
剤形
- 注射
- 解決
- 錠剤
- その他
年齢層
- 小児科
- アダルト
- 老年病
エンドユーザー
- 病院
- 専門クリニック
- ホームケア
- その他
流通チャネル
- 病院薬局
- 小売薬局
- オンライン薬局
- その他
中東およびアフリカの アダリムマブ市場の地域分析/洞察
中東およびアフリカのアダリムマブ市場が分析され、市場規模の洞察と傾向が、上記のように国、薬物クラス、タイプ、適応症、剤形、投与強度、薬物タイプ、投与経路、年齢層、エンドユーザー、流通チャネル別に提供されます。
中東およびアフリカのアダリムマブ市場レポートで取り上げられている国は、中東およびアフリカ (MEA) の一部であるサウジアラビア、UAE、南アフリカ、エジプト、イスラエル、その他の中東およびアフリカ (MEA) です。
サウジアラビアは、この地域における関節炎疾患の負担を克服するための研究開発活動が増加しているため、アダリムマブ市場を独占しています。
レポートの国別セクションでは、市場の現在および将来の傾向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。下流および上流のバリュー チェーン分析、技術動向、ポーターの 5 つの力の分析、ケース スタディなどのデータ ポイントは、個々の国の市場シナリオを予測するために使用される指標の一部です。また、中東およびアフリカのブランドの存在と可用性、および地元および国内ブランドとの競争が激しいか少ないために直面する課題、国内関税と貿易ルートの影響を考慮しながら、国別データの予測分析を提供します。
競争環境と中東・アフリカの アダリムマブ市場シェア分析
中東およびアフリカのアダリムマブ市場の競争状況は、競合他社ごとに詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、生み出される収益、市場の可能性、研究開発への投資、新しい市場への取り組み、中東およびアフリカでのプレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性などがあります。提供されている上記のデータ ポイントは、中東およびアフリカのアダリムマブ市場に関連する会社の焦点にのみ関連しています。
中東およびアフリカのアダリムマブ市場で活動している主要企業は次のとおりです。
- マイランNV(米国)
- ジダス・カディラ(インド)
- ベーリンガーインゲルハイムインターナショナルGmbH(ドイツ)
- アッヴィ社(米国)
- アボット(米国)
- ヘテロバイオファーマ株式会社(インド)
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2 MG/0.4ML
13.3 MG/0.8ML
13.4 MG/0.4ML
13.5 MG/0.1ML
13.6 OTHERS
14 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 MIDDLE EAST & AFRICA
19.1.1 SAUDI ARABIA
19.1.2 SOUTH AFRICA
19.1.3 UAE
19.1.4 ISRAEL
19.1.5 KUWAIT
19.1.6 EGYPT
19.1.7 REST OF MIDDLE EAST & AFRICA
20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
表のリスト
LIST OF TABLES
TABLE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 SAUDI ARABIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 SAUDI ARABIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 SAUDI ARABIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 SAUDI ARABIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 SAUDI ARABIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 SAUDI ARABIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 SAUDI ARABIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 SAUDI ARABIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 SAUDI ARABIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 SAUDI ARABIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 SOUTH AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 SOUTH AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 SOUTH AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 SOUTH AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 SOUTH AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 SOUTH AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 SOUTH AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 SOUTH AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 SOUTH AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 SOUTH AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 UAE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 UAE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 UAE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 UAE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 UAE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 UAE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 UAE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 UAE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 UAE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 UAE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 ISRAEL ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 ISRAEL ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 ISRAEL BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 ISRAEL ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 ISRAEL ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 ISRAEL GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 ISRAEL ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 ISRAEL ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 ISRAEL ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 ISRAEL ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 KUWAIT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 KUWAIT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 KUWAITBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 KUWAIT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 KUWAIT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 KUWAIT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 KUWAIT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 KUWAIT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 KUWAIT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 KUWAIT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 EGYPT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 EGYPT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 EGYPT BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 EGYPT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 EGYPT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 EGYPT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 EGYPT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 EGYPT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 EGYPT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 EGYPT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 REST OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
図表一覧
LIST OF FIGURES
FIGURE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。