世界の使い捨て医療機器再処理市場の規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
4.36 Million
USD
14.71 Million
2024
2032
USD
4.36 Million
USD
14.71 Million
2024
2032
| 2025 –2032 | |
| USD 4.36 Million | |
| USD 14.71 Million | |
|
|
|
|
世界の使い捨て医療機器再処理市場のセグメンテーション、製品タイプ(クラスI機器およびクラスII機器)、価格帯(高価格帯および低価格帯)、用途(一般外科、麻酔、関節鏡検査および整形外科、心臓病学、消化器学、泌尿器科、婦人科、その他)、タイプ(社内およびアウトソース)、エンドユーザー(病院、 外来手術センター、その他)、流通チャネル(B2BおよびB2C) - 2032年までの業界動向および予測
使い捨て医療機器の再処理市場規模
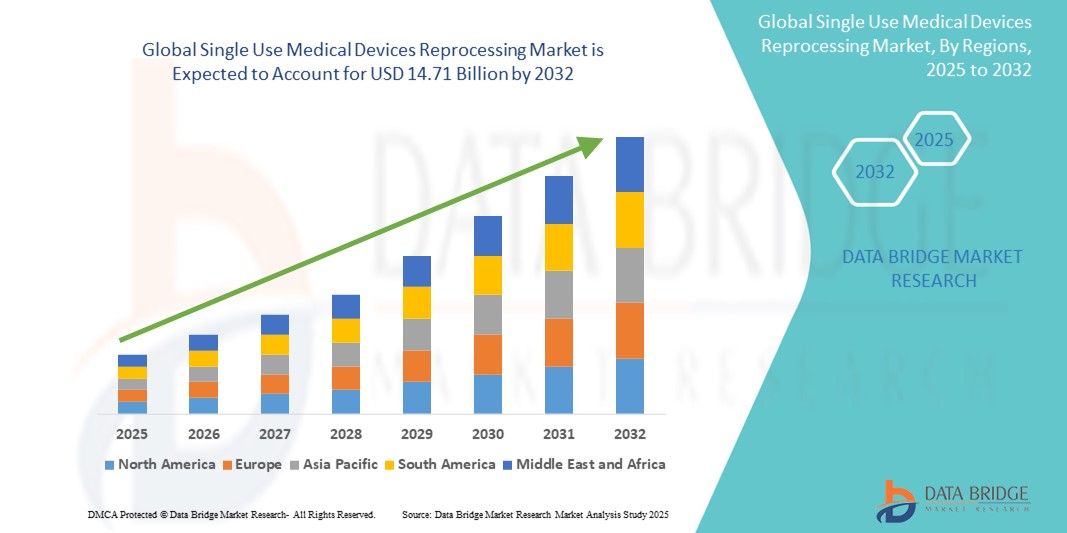
- 世界の使い捨て医療機器の再処理市場規模は2024年に43億6000万米ドルと評価され、予測期間中に16.40%のCAGRで成長し、2032年には147億1000万米ドル に達すると予想されています 。
- 市場の成長は、費用対効果の高いヘルスケアソリューションの採用の増加と再処理技術の技術的進歩によって主に推進されており、病院と臨床の両方の環境で使い捨て医療機器の再処理の受け入れが増加しています。
- さらに、持続可能で規制に準拠し、環境に配慮した医療行為への需要の高まりにより、使い捨て医療機器の再処理は、医療廃棄物の削減と資源利用の最適化を実現する現実的なソリューションとして確立されつつあります。これらの要因が重なり、使い捨て医療機器の再処理ソリューションの普及が加速し、業界の成長を大きく後押ししています。
使い捨て医療機器の再処理市場分析
- 使い捨て医療機器の再処理は、使い捨てラベルの付いた機器の洗浄、滅菌、試験、再包装を含み、その費用効率、安全性、環境面での利点により、病院や外科センターにおける現代の医療費抑制戦略においてますます重要な要素になりつつあります。
- 使い捨て医療機器の再処理に対する需要の高まりは、主に医療提供者に対するコスト削減の圧力の高まり、医療における環境持続可能性への意識の高まり、安全な再処理の実践を促進する好ましい規制枠組みによって促進されています。
- 北米は、2024年には42.6%という最大の収益シェアで使い捨て医療機器の再処理市場を席巻しました。これは、広範な医療インフラ、再処理プロトコルの早期導入、そして主要な市場プレーヤーの存在を特徴としています。米国は、コスト効率化対策とFDAガイドラインへの厳格な遵守を背景に、再処理機器の利用が大幅に増加し、この地域をリードしています。
- アジア太平洋地域は、医療費の増加、医療廃棄物の管理における再処理の採用増加、中国やインドなどの新興経済国における私立病院ネットワークの拡大により、予測期間中に使い捨て医療機器の再処理市場において17.5%のCAGRで最速の成長を遂げる地域になると予想されています。
- クラスIIデバイスセグメントは、2024年に57.4%の市場シェアで使い捨て医療機器の再処理市場を支配しました。これは、これらのデバイスが医療提供者に提供する高い再処理能力と大幅なコスト削減、特に心臓病学や整形外科などの大量専門分野に起因しています。
レポートの範囲と使い捨て医療機器の再処理市場のセグメンテーション
|
属性 |
使い捨て医療機器の再処理に関する主要な市場洞察 |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
使い捨て医療機器の再処理市場の動向
「再処理プロトコルの効率と自動化の向上」
- 世界のシングルユース医療機器の再処理市場において、インテリジェントな自動化技術とデジタル追跡システムの統合による再処理ワークフローの合理化と規制遵守の確保が、重要かつ加速しているトレンドとなっています。こうした技術の融合により、医療施設全体の運用効率とトレーサビリティが大幅に向上しています。
- 例えば、高度な再処理システムには、バーコードやRFIDによる自動ログ記録と機器追跡機能が搭載されており、病院は各機器の再処理サイクルを監視し、安全基準を満たしていることを確認できます。同様に、スマート滅菌ユニットは、技術者にリアルタイムのデータとアラートを提供することで、精度を確保し、手作業によるエラーを最小限に抑えることができます。
- 再処理ワークフローに統合されたデジタルツールは、使用パターンを学習し、サイクルタイミングとリソース割り当ての最適化を支援します。これにより、ターンアラウンドタイムが短縮され、デバイスのダウンタイムが削減されます。例えば、一部の集中型再処理センターでは、ソフトウェア駆動型システムを活用してメンテナンススケジュールを予測し、ボトルネックを削減しています。さらに、自動アラートとリアルタイムダッシュボードにより、医療管理者は情報に基づいた迅速な意思決定が可能になります。再処理ユニットと病院管理システムのシームレスな統合により、滅菌、在庫、使用データの集中管理が容易になります。医療提供者は、単一のインターフェースを通じて、再処理されたデバイスのライフサイクル全体を管理し、コスト削減を追跡し、規制プロトコルの遵守を確保できます。
- よりインテリジェントで直感的、かつ相互接続された再処理システムへのトレンドは、病院における医療機器の再利用へのアプローチを根本的に変革しています。その結果、企業は、品質、安全性、そしてコスト効率に対する高まる需要に応えるため、追跡、分析、そして自動検証システムを備えた再処理技術を開発しています。
- 医療システムが持続可能性、コンプライアンス、運用効率をますます重視するにつれて、病院のITインフラストラクチャとのシームレスな統合を提供する再処理ソリューションの需要は、先進市場と新興市場の両方で急速に高まっています。
使い捨て医療機器の再処理市場の動向
ドライバ
「医療施設におけるセキュリティ上の懸念の高まり」
- 医療施設におけるセキュリティ上の懸念の高まりと、高度な医療インフラの導入の加速は、使い捨て医療機器の再処理市場における需要の高まりの大きな原動力となっています。
- 例えば、2024年4月、オニティ社(ハネウェル・インターナショナル社)は、IoTベースのセルフストレージセキュリティの進化を発表し、パスポートロックソリューションに最先端のセンサーを統合することを目指しています。主要企業によるこのような戦略は、予測期間中の使い捨て医療機器再処理市場の成長を牽引すると予想されます。
- 病院や診療所が医療に関連する潜在的なリスクをより意識するようになり、費用対効果が高く持続可能な対策を通じて保護の強化を求めるようになるにつれて、再処理された使い捨て医療機器は、品質保証、規制遵守、環境への影響の軽減などの高度な利点を提供し、新しい使い捨て機器に代わる魅力的な選択肢となります。
- さらに、持続可能な医療ソリューションの人気が高まり、医療現場におけるコスト抑制への要望が高まるにつれ、再生医療機器は現代の病院調達戦略において不可欠な要素となっています。これらの機器は既存のシステムとのシームレスな互換性を提供し、より環境に配慮した医療業務を促進します。
- 低コスト、医療廃棄物の最小化、そして性能を犠牲にすることなく規制基準を遵守できるという利便性は、公的医療分野と民間医療分野の双方において、再処理されたシングルユース医療機器の導入を促進する重要な要因です。環境に配慮した慣行への傾向と、ユーザーフレンドリーなシングルユース医療機器の再処理ソリューションの利用可能性の増加は、市場の成長をさらに促進しています。
抑制/挑戦
「サイバーセキュリティと初期コストの高さへの懸念」
- 再処理管理プラットフォームを含む、接続された医療システムのサイバーセキュリティの脆弱性に関する懸念は、市場へのより広範な浸透にとって大きな課題となっています。使い捨て医療機器の再処理市場のソリューションの多くは、使用状況とコンプライアンスを追跡するためにネットワーク接続とソフトウェアに依存しているため、ハッキング攻撃やデータ漏洩の影響を受けやすく、病院や規制当局の間でデータプライバシーと患者の安全性に関する不安が高まっています。
- 例えば、医療IoTシステムの脆弱性に関する注目を集めた報告により、一部の施設では病院のITネットワークと統合された高度な再処理および追跡システムの導入をためらうようになっている。
- 医療機関間の信頼を築くには、堅牢な暗号化、安全な認証プロトコル、定期的なソフトウェアアップデートなどを通じて、こうしたサイバーセキュリティ上の懸念に対処することが不可欠です。Stryker Sustainability SolutionsやVanguard AGといった企業は、潜在顧客の安心感を高めるため、マーケティングにおいて安全でコンプライアンスに準拠したインフラを強調しています。
- さらに、一部の先進的なシングルユース医療機器の再処理市場システムは、従来の調達プロセスと比較して初期コストが比較的高いため、特に発展途上地域や小規模な診療所など、コストに敏感な医療機関にとって導入の障壁となる可能性があります。基本的な再処理オプションはより手頃な価格になっていますが、自動追跡、品質検証、ロボット洗浄システムなどのプレミアム機能は、多くの場合、より高額です。
- コストは徐々に低下しているものの、持続可能で高度な再処理技術に対する認識されたプレミアムは、特に長期的なコスト削減と環境上の利点をすぐに認識できないプロバイダーにとって、依然として広範な導入を妨げる可能性があります。
- サイバーセキュリティ対策の強化、持続可能な医療実践に関する教育、より手頃な価格の使い捨て医療機器再処理市場ソリューションの開発を通じてこれらの課題を克服することが、持続的な成長に不可欠です。
使い捨て医療機器の再処理市場の範囲
世界の使い捨て医療機器再処理市場は、製品タイプ、価格帯、用途、タイプ、エンドユーザー、流通チャネルに基づいて区分されています。
• 製品タイプ別
製品タイプに基づいて、使い捨て医療機器の再処理市場はクラスI機器とクラスII機器に分類されます。クラスII機器セグメントは、高い再処理能力と医療提供者への大幅なコスト削減効果により、2024年には57.4%と最大の市場収益シェアを占めました。
クラス I デバイスセグメントは、より単純な機器の再処理の受け入れ拡大により、2025 年から 2032 年にかけて 19.2% という最も高い CAGR を達成すると予想されています。
• 価格帯別
価格帯に基づいて、使い捨て医療機器の再処理市場は、ハイレンジとロー/エコノミーレンジに分類されます。ロー/エコノミーレンジセグメントは、中小規模の医療施設からの高い需要により、2024年には61.8%という最大の市場収益シェアを占めました。
ハイレンジセグメントは、高級再加工製品の需要増加に支えられ、2025年から2032年にかけて17.5%という最も高いCAGRで成長すると予測されています。
• アプリケーション別
用途別に見ると、使い捨て医療機器の再処理市場は、一般外科、麻酔科、関節鏡検査・整形外科、心臓病学、消化器科、泌尿器科、婦人科、その他に分類されます。再処理可能な器具の頻繁な使用により、一般外科分野は2024年に26.9%と最大の収益シェアを占めました。
心臓病学分野は、2025年から2032年にかけて20.1%という最も高いCAGRを記録し、手術件数の増加、コスト削減、心血管疾患の有病率が見込まれています。
使い捨て医療機器の再処理市場の地域分析
- 北米は、広範囲にわたる医療インフラ、再処理プロトコルの早期導入、主要な市場プレーヤーの存在を特徴とし、2024年には42.6%という最大の収益シェアで使い捨て医療機器の再処理市場を支配しました。
- 米国は、コスト効率対策とFDAガイドラインの厳格な遵守により、再処理機器の利用が大幅に増加し、この地域をリードしています。
- この地域は、FDAの支援的なガイドラインと医療廃棄物の削減への関心の高まりの恩恵を受けており、病院や外科センターでは持続可能性と費用効率のために再処理された使い捨て機器を採用するよう奨励されています。
米国における使い捨て医療機器の再処理市場の洞察
米国の使い捨て医療機器再処理市場は、2024年に北米市場の収益シェアの80%を占め、同地域最大の市場となりました。この成長は、費用対効果の高い手術器具に対する病院の需要の高まり、強力なサードパーティ再処理企業、そしてクラスIおよびクラスII機器の再処理に対する規制当局の支援によって牽引されています。さらに、米国では病院がコスト管理と環境持続可能性を優先していることから、院内および外部委託による再処理プログラムの導入率が高くなっています。
欧州における使い捨て医療機器の再処理市場の洞察
欧州の使い捨て医療機器の再処理市場は、医療費抑制と持続可能性に関する規制の強化を主な原動力として、予測期間を通じて大幅なCAGRで拡大すると予測されています。欧州各国は、EUの廃棄物削減目標を達成するために再処理ソリューションを導入しており、特に循環型経済の実践に取り組む病院でその需要が高まっています。再処理されたカテーテルや整形外科用器具がバリデーション後に広く再利用されているため、外科部門では需要が顕著です。
英国の使い捨て医療機器の再処理市場に関する洞察
英国の使い捨て医療機器の再処理市場は、NHSとサードパーティの再処理業者との提携拡大や持続可能な医療慣行への投資増加に支えられ、予測期間中に注目すべきCAGRで成長すると予想されています。機器ライフサイクル管理に関する意識の高まりとMHRA(英国保健省)による規制の明確化は、病院が調達コストの削減を目指して再処理を導入することを後押ししています。
ドイツにおける使い捨て医療機器の再処理市場の洞察
ドイツの使い捨て医療機器の再処理市場は、堅牢な医療システム、高度な技術革新、そして医療機器の再処理基準(DIN EN ISO 13485など)への厳格な遵守に牽引され、予測期間中に大幅なCAGRで拡大すると予想されています。病院や診療所では、臨床パフォーマンスを維持しながら運用コストを削減するため、再処理済みの心臓外科用機器や整形外科用機器を選択するケースが増えています。
アジア太平洋地域の使い捨て医療機器再処理市場に関する洞察
アジア太平洋地域の使い捨て医療機器再処理市場は、2025年から2032年にかけて17.5%という最も高い年平均成長率(CAGR)で成長すると予測されています。この地域の成長は、持続可能な医療慣行への意識の高まり、コストに配慮した医療システム、そしてインドや中国などの国々における政府の好ましい政策によって牽引されています。民間病院の急速な拡大と、サードパーティによる再処理サービスの導入増加は、市場の大きな潜在的可能性に貢献しています。
日本における使い捨て医療機器の再処理市場に関する洞察
日本の使い捨て医療機器の再処理市場は、ハイテク医療インフラ、人口の高齢化、そして医療機器の安全性と再利用への強い関心に牽引され、2024年にはアジア太平洋地域の市場シェアの18.5%を占めました。再処理された外科用および診断用機器は、医療廃棄物の削減と国の持続可能性目標への適合を目的として、三次医療機関で広く利用されています。
中国における使い捨て医療機器の再処理市場の洞察
中国の使い捨て医療機器の再処理市場は、医療改革の進展、急速な都市化、そして強力な国内製造能力により、2024年にはアジア太平洋地域において42.6%の市場シェアを獲得し、圧倒的なシェアを占める見込みです。費用対効果の高い医療サービスの提供への取り組みと、一級都市における集中的な再処理拠点の整備が相まって、公立病院と私立病院の両方で再処理の導入が加速しています。
使い捨て医療機器の再処理市場シェア
使い捨て医療機器の再処理業界は、主に、次のような老舗企業によって主導されています。
- ジョンソン・エンド・ジョンソン・サービス社(米国)
- ストライカー(米国)
- メドライン・インダストリーズ社(米国)
- イノベーティブヘルス(米国)
- アルジョ(スウェーデン)
- シュアテック・メディカル(米国)
- ヴァンガードAG(米国)
- NEScientific, Inc.(米国)
- ReNu Medical(米国)
- ジェイドライフサイエンスプライベートリミテッド(インド)
世界の使い捨て医療機器再処理市場の最新動向
- 2023年2月、米国に拠点を置く再処理業者であるNortheast Scientific Inc.は、Philips IVUS Eagle Eye Platinum RXデジタルカテーテルの再処理についてFDA 510(k)認可を取得しました。これにより、本カテーテルはFDA承認を受けた初の再処理済みデジタル血管内超音波カテーテルとなり、臨床性能を損なうことなくカテーテル検査室の大幅なコスト削減を実現します。
- 2022年6月、医療機器再処理業者協会(AMDR)は、「使い捨て医療機器の再処理および再製造に関する国際規制基準」を発表しました。この包括的なロードマップは、国際規制を調和させ、19か国以上の規制機関が医療システムに再処理を安全に統合できるようにします。
- デンマークは2025年1月1日、MDR第17条に基づく使い捨て医療機器の再処理と再利用を正式に認める改正大統領令を制定しました。この規制は再処理業者によるCEマークの取得を義務付け、デンマークを持続可能な再処理慣行を推進する早期導入国の一つに位置付けています。
- 2024年11月、欧州委員会はEU加盟国におけるMDR第17条の実施状況に関する最初の報告書を提出しました。この評価では、各国が国内法に基づき、使い捨て機器の再処理に関する安全で準拠した枠組みをどのように採用しているかが強調されています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。