世界の規制関連業務アウトソーシング市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
11.49 Billion
USD
25.85 Billion
2024
2032
USD
11.49 Billion
USD
25.85 Billion
2024
2032
| 2025 –2032 | |
| USD 11.49 Billion | |
| USD 25.85 Billion | |
|
|
|
|
世界の薬事アウトソーシング市場セグメント、サービス展望(薬事コンサルティング、法的代理、薬事文書作成および発行、製品登録、臨床試験申請およびその他のサービス)、規模(小規模、中規模、大規模)、カテゴリ(医薬品、ジェネリック、イノベーター、生物製剤、バイオテクノロジー、ATMP、医療機器、治療および診断)、適応症(腫瘍学、神経学、心臓学、免疫学およびその他)、段階(前臨床、臨床およびPMA(市販後承認)、エンドユーザー(医療機器企業、製薬企業、バイオテクノロジー企業)別 - 2032年までの業界動向および予測
規制関連業務アウトソーシング市場規模
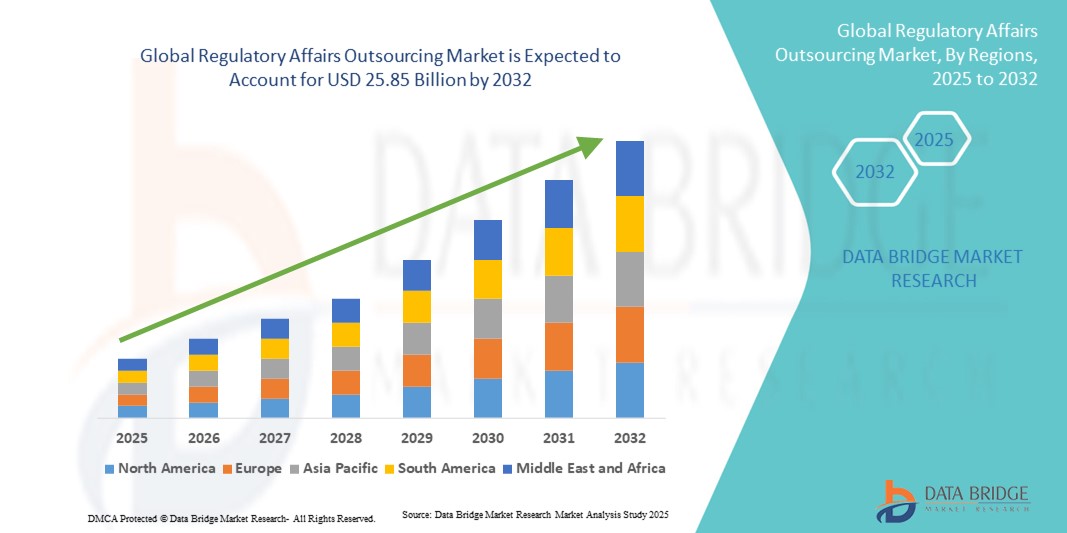
- 世界の規制業務アウトソーシング市場規模は2024年に114億9000万米ドルと評価され、予測期間中に10.66%のCAGRで成長し、2032年には258億5000万米ドル に達すると予想されています 。
- 市場の成長は、製薬、バイオテクノロジー、医療機器業界における規制要件の複雑化によって主に促進されており、企業はコンプライアンス管理のための専門的なアウトソーシングサービスを求めるようになっています。
- さらに、費用対効果が高く、効率的で、タイムリーな規制申請に対する需要の高まりが、規制関連業務アウトソーシングソリューションの導入を促進しています。これらの要因が重なり、規制関連業務アウトソーシングサービスの導入が加速し、業界の世界的な成長を大きく後押ししています。
規制業務アウトソーシング市場分析
- コンプライアンス、申請、文書化業務を専門のサービスプロバイダーに委託する規制業務アウトソーシングは、複雑な国際規制を効率的に乗り越えることを目指す製薬、バイオテクノロジー、医療機器企業にとって不可欠な要素になりつつあります。
- 規制に関する専門知識への需要の高まりと、地域をまたぐ承認プロセスの複雑化が相まって、規制関連業務アウトソーシングサービスの導入が進んでいます。企業はこれらのサービスを活用し、タイムリーな市場アクセスの確保、コンプライアンスリスクの軽減、そして中核となる研究開発活動への集中を実現しています。
- 北米は、大手製薬企業やバイオテクノロジー企業の存在、厳格なFDA規制、そして専門的な規制サポートを必要とする臨床試験の多さに牽引され、2024年には規制関連業務アウトソーシング市場において40.5%という最大の収益シェアを獲得し、市場を席巻しました。米国は、整備されたインフラ、広範な規制ガイドライン、そして専門家によるアウトソーシングソリューションへの高い需要により、この地域の市場をリードしています。
- アジア太平洋地域は、予測期間中、医薬品製造の拡大、ヘルスケア投資の増加、そして中国、インド、日本などの国々における規制遵守への意識の高まりを背景に、規制関連業務アウトソーシング市場において最も急速な成長を遂げる地域になると予想されています。費用対効果の高いアウトソーシングソリューションと増加する臨床試験活動が、市場の成長をさらに加速させます。
- 大規模セグメントは、2024年には45%という最大の収益シェアで規制関連業務アウトソーシング市場を席巻しました。これは、多国籍製薬、バイオテクノロジー、医療機器企業の広範なグローバル展開に支えられています。大規模組織は、大量の製品登録の管理、複数地域にわたるコンプライアンスの維持、業務効率の最適化のために、規制関連業務をアウトソーシングすることがよくあります。
レポートの範囲と規制業務アウトソーシング市場のセグメンテーション
|
属性 |
規制関連業務アウトソーシングの主要市場インサイト |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
規制関連業務アウトソーシング市場の動向
規制に関する専門知識に対する需要の高まり
- 世界的な規制関連業務アウトソーシング市場において、複雑な規制申請、コンプライアンス関連文書、承認プロセスの管理において、専門サービスプロバイダーへの依存度が高まっていることは、重要かつ加速するトレンドです。このトレンドは、複数の地域におけるタイムリーな市場アクセスと、変化する規制への遵守の必要性によって推進されています。
- 製薬、バイオテクノロジー、医療機器企業は、業務の効率化、コンプライアンスリスクの軽減、そして中核となる研究開発活動への注力を目的として、規制関連業務のアウトソーシングをますます進めています。
- 市場の主要なサービスプロバイダーは、書類作成、申請管理、市販後調査、規制情報など、エンドツーエンドの規制サポートを含むサービスを拡大し、クライアントが地域および世界の要件に準拠し続けることを保証しています。
- 規制の複雑さが増し、FDA、EMA、PMDAなどの当局からの厳格なガイドラインが相まって、グローバルな規制戦略の専門知識とより迅速な申請スケジュールを提供するアウトソーシングソリューションの需要が高まっています。
- さらに、北米、欧州、アジア太平洋地域での臨床試験活動の増加と製品承認数の増加が市場の成長を促しており、企業はコスト効率が高く信頼性の高い規制サポートサービスを求めています。
- 規制業務アウトソーシング市場は、大手製薬企業と中小企業の両方で拡大しており、その幅広い採用と、製品開発と商業化の加速におけるアウトソーシングされた専門知識の重要な役割が浮き彫りになっています。
規制関連業務アウトソーシング市場の動向
ドライバ
規制の複雑化とグローバル展開によるニーズの高まり
- 製薬、バイオテクノロジー、医療機器業界における規制要件の複雑化と、ヘルスケア市場の世界的な拡大が相まって、規制業務のアウトソーシングに対する需要の高まりの大きな要因となっています。
- 例えば、2024年4月には、大手CROが、グローバル市場向けにAIを活用した申請追跡とコンプライアンス監視を含む規制コンサルティングサービスの拡充を発表しました。主要企業によるこのような戦略は、予測期間中の規制関連業務アウトソーシング業界の成長を牽引すると予想されます。
- 製薬会社やバイオテクノロジー会社は、より厳格なコンプライアンス基準や規制枠組みの頻繁な更新に直面しており、アウトソーシングは、製品登録、臨床試験申請、市販後承認に関する専門知識へのアクセスを提供し、社内管理に代わる魅力的な選択肢を提供しています。
- さらに、世界的な製品発売や国境を越えた臨床試験の増加傾向により、規制業務のアウトソーシングは業務戦略の不可欠な部分となり、企業は多様な規制環境を効率的にナビゲートし、タイムリーな承認を確保できるようになりました。
- 経験豊富な規制専門家の活用、社内コンプライアンス業務の負荷軽減、そして変化する規制に関する最新のガイダンスへのアクセスといった利便性は、大手製薬会社と新興バイオテクノロジー企業の両方において、規制関連業務のアウトソーシング導入を推進する重要な要因となっています。戦略的パートナーシップへの傾向と、柔軟なアウトソーシングオプションの利便性向上も、市場の成長に寄与しています。
抑制/挑戦
データセキュリティと高額なサービスコストに関する懸念
- 機密性の高い臨床データや規制データの機密性とセキュリティに関する懸念は、規制関連業務のアウトソーシングの普及拡大にとって大きな課題となっています。アウトソーシングは外部のサービスプロバイダーやデジタルプラットフォームに依存するため、データ漏洩や不正アクセスの潜在的なリスクがあり、コンプライアンスや知的財産保護に関する企業の不安を高めています。
- 例えば、データ管理プラットフォームにおけるサイバーセキュリティの脆弱性に関する報告により、一部の企業は規制機能のアウトソーシングに慎重になっている。
- 堅牢な暗号化、安全な認証プロトコル、そして国際的なデータ保護基準(GDPR、HIPAAなど)への準拠を通してこれらの懸念に対処することは、顧客の信頼を築く上で不可欠です。ParexelやMedpaceといった企業は、潜在的な顧客に安心感を与えるために、サービス提供において高度なデータセキュリティ対策とコンプライアンスフレームワークを強調しています。
- さらに、包括的な規制アウトソーシング サービスのコストは社内チームに比べて比較的高いため、特に新興市場では中小企業にとって障壁となる可能性があります。
- サービス料金は徐々に競争力を増しているものの、規制に関する専門知識をアウトソーシングすることに対するプレミアム感が、特に予算が限られている企業や小規模な製品ポートフォリオを管理する企業にとって、導入を阻む要因となっている。
- 強化されたデータセキュリティプロトコル、コンプライアンスのベストプラクティスに関する顧客教育、費用対効果の高い規制アウトソーシングソリューションの開発を通じてこれらの課題を克服することは、持続的な市場成長にとって不可欠です。
規制業務アウトソーシング市場の範囲
市場は、サービスの見通し、規模、カテゴリ、適応症、段階、およびエンドユーザーに基づいてセグメント化されています。
- サービス展望別
サービス展望に基づき、市場は規制コンサルティング、法的代理、規制文書の作成と公開、製品登録、臨床試験申請、その他のサービスに分類されています。規制コンサルティングセグメントは、2024年に42.5%という最大の収益シェアで市場を支配しました。この優位性は、製薬企業とバイオテクノロジー企業が、複雑なグローバル規制の枠組みを乗り越えるために専門家のガイダンスにますます依存していることに起因しています。規制コンサルティングは、書類作成、規制申請、コンプライアンス管理のための戦略的サポートを提供し、承認の迅速化と却下リスクの低減を保証します。このセグメントの強力な存在感は、製品パイプラインの増加、新薬の発売、研究開発投資の増加によって強化されています。多国籍企業は、複数地域の規制に関する知識とカスタマイズされたソリューションを提供できるため、コンサルティングサービスを好む傾向があります。変化する規制環境でコンプライアンスを維持する必要性により、規制コンサルティングは主要サービスとしての地位をさらに強固なものにしています。
臨床試験申請セグメントは、臨床試験申請の複雑化と厳格な規制の強化を背景に、2025年から2032年にかけて22.1%という最も高いCAGRを達成すると予想されています。臨床試験申請のアウトソーシングにより、企業は申請を効率的に管理し、国際ガイドラインを遵守し、複数の地域での承認を追跡することが可能になります。腫瘍学、神経学、免疫学の試験件数の増加と、デジタル申請プラットフォームの導入拡大が相まって、需要が高まっています。企業は、リスクの最小化、効率性の向上、そして正確性の確保のために、アウトソーシングを好んでいます。また、大規模な社内規制チームを持たずに専門知識を求める中小企業も成長を支えており、このセグメントの高い潜在性を浮き彫りにしています。
- サイズ別
市場規模に基づき、小規模、中規模、大規模の3つに分類されます。大規模セグメントは、多国籍製薬、バイオテクノロジー、医療機器企業の広範なグローバル展開を背景に、2024年には45%という最大の収益シェアで市場を席巻しました。大規模組織は、大量の製品登録の管理、複数地域にわたるコンプライアンスの維持、業務効率の最適化のために、規制業務をアウトソーシングすることがよくあります。強力な財務および技術リソースを備えたこれらの企業は、複雑な規制業務を処理できるトップクラスのサービスプロバイダーと提携することができます。また、大企業は規制の遅延リスクの軽減というメリットも享受し、中核となる研究開発や戦略的優先事項に集中することができます。パイプラインの拡大、複数の製品の発売、そして厳格なグローバル規制により、大企業における規制業務アウトソーシングの需要は増加し続けています。
新興バイオテクノロジー企業や新興製薬企業がアウトソーシングへの依存度を高めていることから、小規模企業セグメントは2025年から2032年にかけて20.8%という最も高いCAGR(年平均成長率)を記録すると予測されています。小規模企業は多くの場合、社内に大規模な規制チームを擁しておらず、製品登録、コンプライアンス、臨床試験申請において外部の専門知識を活用する傾向があります。このセグメントの成長は、規制要件に関する意識の高まり、臨床試験件数の増加、そして費用対効果の高いアウトソーシングソリューションによって支えられています。この傾向は、社内に規制に関する知識が限られている新興バイオテクノロジーハブを抱える地域で特に顕著です。アウトソーシングは、小規模企業が経験豊富な専門家にアクセスし、多額の運用投資をすることなく迅速な承認取得を可能にします。
- カテゴリー別
カテゴリー別に見ると、市場は医薬品、ジェネリック医薬品、イノベーター、生物製剤、バイオテクノロジー、ATMP、医療機器、治療薬、診断薬に分類されています。医薬品セグメントは、新規化学物質の数が多く、広範な規制申請が必要であることから、2024年には41.7%という最大の収益シェアで市場を支配しました。このセグメントにおける規制アウトソーシングにより、企業はFDA、EMA、PMDAのガイドラインへの準拠を維持しながら、市場投入までの時間を短縮することができます。企業は、専門家による文書管理、グローバルな申請戦略、承認追跡の恩恵を受けています。この優位性は、多額の研究開発投資、大規模な製品パイプライン、そして世界中で増加する承認数によって強化されています。アウトソーシングは、申請エラーや規制の遅延のリスクを軽減するのに役立ち、このセグメントを市場成長の重要な原動力にしています。
ATMP(先進治療医薬品)セグメントは、遺伝子・細胞治療のイノベーションに牽引され、2025年から2032年にかけて23.4%という最も高いCAGR(年平均成長率)を達成すると予想されています。規制アウトソーシングサービスは、複雑な文書作成、臨床試験申請、市販後コンプライアンスに関する専門知識を提供します。研究開発投資の増加、承認件数の増加、そして先進治療における規制の複雑さにより、外部からのサポートが不可欠となっています。企業はアウトソーシングを活用することで、複数地域にまたがる規制を効率的に回避し、製品の迅速な発売とコンプライアンス強化を実現しています。腫瘍学、免疫学、希少疾患におけるATMPの採用は、このセグメントの成長をさらに加速させます。
- 適応症別
適応症に基づいて、市場は腫瘍学、神経学、心臓学、免疫学、その他に分類されます。腫瘍学分野は、がん治療への世界的な研究開発投資の増大と腫瘍学治療薬の承認件数の増加により、2024年には44.2%という最大の収益シェアを占めると予測されます。腫瘍学における規制アウトソーシングは、企業が申請量、複雑な文書管理、複数国へのコンプライアンスを効率的に管理するのに役立ちます。がんの罹患率の増加と標的療法の革新は、アウトソーシングの需要を刺激しています。この分野は、強力な政府の支援、迅速な承認プロセス、大規模な臨床試験の恩恵を受けており、最も収益性の高い適応症として位置付けられています。アウトソーシングは、規制が厳しく競争の激しい腫瘍学市場において、承認の迅速化とリスクの低減を可能にします。
免疫学分野は、2025年から2032年にかけて、新規免疫療法、ワクチン、生物製剤の開発に牽引され、21.6%という最も高い年平均成長率(CAGR)を達成すると予測されています。規制関連アウトソーシングは、新興治療法の治験申請、文書作成、そして規制遵守に関する専門知識を提供します。免疫学の研究開発への意識の高まり、投資の増加、そして革新的な生物製剤の導入が成長を牽引しています。企業は、規制の効率性を確保しながら、社内リソースの制約を克服するためにアウトソーシングを活用しています。この分野の急速な成長は、世界市場の拡大と高度な免疫学的治療に対する需要の高まりにも支えられています。
- ステージ別
市場は段階別に、前臨床、臨床、PMA(市販後承認)に分類されます。臨床段階は、世界的な臨床試験の件数の増加と厳格な規制遵守要件に牽引され、2024年には43%という最大の収益シェアを占めると予測されています。臨床段階の業務をアウトソーシングすることで、企業は地域をまたいで試験プロトコル、申請、承認を効率的に管理できます。腫瘍学、神経学、心臓病学の試験に対する高い需要も成長を後押ししています。企業は、迅速な規制審査、正確な文書化、経験豊富な専門家へのアクセスといったメリットを享受し、業務負担を軽減できます。臨床段階の規制業務の複雑さと量により、この分野は引き続き市場を牽引する主要な要因となっています。
PMA(市販後承認)セグメントは、市販後調査の要件、有害事象報告、医薬品および医療機器の継続的なコンプライアンス義務の増加により、2025年から2032年にかけて20.9%という最も高いCAGRを達成すると予想されています。PMA関連業務のアウトソーシングにより、企業は安全性監視の徹底、文書の更新、そして進化する規制への効率的なコンプライアンス遵守を実現できます。バイオ医薬品、医療機器、先進治療の承認件数の増加も成長を支えており、PMAアウトソーシングは持続可能なコンプライアンスの実現に不可欠です。
- エンドユーザー別
エンドユーザー別に見ると、市場は医療機器企業、製薬企業、バイオテクノロジー企業に分類されます。製薬企業セグメントは、多数の医薬品承認、広範な臨床試験、そして世界的な規制要件に支えられ、2024年には46%という最大の市場収益シェアを占めると予測されます。アウトソーシングにより、製薬企業は国際規制へのコンプライアンスを確保しながら、コアとなる研究開発に集中することができます。企業はリスク軽減、運用コストの削減、そして製品発売の迅速化といったメリットを享受できます。この優位性は、大規模なグローバルオペレーションと複数の製品パイプラインによって強化されており、製薬企業は規制アウトソーシングサービスの最大のユーザーとなっています。
バイオテクノロジー企業セグメントは、遺伝子治療、生物製剤、その他のバイオテクノロジー製品における急速なイノベーションに牽引され、2025年から2032年にかけて22.7%という最も高いCAGR(年平均成長率)を達成すると予測されています。規制機能のアウトソーシングは、バイオテクノロジー企業が社内のキャパシティ制約を克服し、複数国への申請を効率的に管理するのに役立ちます。この成長は、バイオテクノロジー研究開発への投資の増加、バイオテクノロジーハブの台頭、そして規制のベストプラクティスに対する意識の高まりによってさらに加速しています。バイオテクノロジー企業は、規制が厳しく、かつ変化の激しい環境において、承認手続きを迅速化し、コンプライアンスを確保するために、専門のサービスプロバイダーに依存しています。
規制業務アウトソーシング市場の地域分析
- 北米は、医薬品、バイオテクノロジー、医療機器分野における専門的な規制サービスに対する需要の増加により、2024年には規制業務アウトソーシング市場において40.5%という最大の収益シェアを占めました。
- この地域の成熟した医療インフラ、主要な世界的なCROの存在、そして革新的な規制戦略の早期導入が、市場リーダーシップに貢献しています。
- 北米の企業は、FDA(米国食品医薬品局)およびカナダ保健省(Health Canada)の厳格な規制へのコンプライアンス確保のため、規制コンサルティング、製品登録、臨床試験申請などのサービスをアウトソーシングするケースが増えています。規制の複雑さに対する高い意識と、確立された法的・技術的サポートが相まって、中小企業から大企業まで、アウトソーシングの導入がさらに加速しています。
米国規制業務アウトソーシング市場インサイト
米国の薬事アウトソーシング市場は、専門的な規制サポートを必要とする医薬品、バイオテクノロジー、医療機器の承認件数の増加に支えられ、2024年には北米で最大の収益シェア(81%)を獲得しました。この市場は、高度な規制枠組み、CROの強力な存在感、そして規制文書作成、法的代理、市販後承認管理といったサービスへの高い需要に支えられています。腫瘍学、神経学、心臓学、免疫学、その他の治療領域の大規模、中規模、小規模の企業は、前臨床、臨床、市販後承認の各段階を効率的に管理するために、アウトソーシングへの依存度を高めています。
欧州規制業務アウトソーシング市場に関する洞察
欧州の薬事アウトソーシング市場は、予測期間中に大幅なCAGRで拡大すると予測されています。これは主に、EU全体の複雑な規制枠組みと、生物製剤、ATMP、医療機器といった革新的治療法のコンプライアンスへの関心の高まりを背景にしています。ドイツ、フランス、その他のEU諸国の企業は、コスト管理とタイムリーな承認取得のため、臨床試験申請、製品登録、薬事コンサルティングなどの薬事関連サービスをアウトソーシングしています。都市化の進展、研究開発活動の活発化、そして専門の薬事関連サービスプロバイダーの存在が、中小企業から大企業まで、市場の成長を促進しています。
英国の規制関連業務アウトソーシング市場に関する洞察
英国の薬事関連業務アウトソーシング市場は、製薬企業およびバイオテクノロジー企業による規制コンプライアンスサービスへの需要増加に牽引され、予測期間中に注目すべきCAGRで成長すると予想されています。規制文書の作成・公開、臨床試験申請管理、製品登録といったアウトソーシングサービスは、企業がMHRA規制を効率的に遵守するのに役立ちます。強固なヘルスケアエコシステム、確立されたCRO、そして規制コンサルティングにおける強力な専門知識が相まって、腫瘍学、心臓病学、免疫学を含む複数の治療領域における成長を支えています。
ドイツの規制関連業務アウトソーシング市場に関する洞察
ドイツの薬事関連アウトソーシング市場は、予測期間中に大幅なCAGRで拡大すると予想されています。これは、規制コンプライアンスへの意識の高まり、厳格な欧州規制、そして前臨床、臨床、市販後承認プロセスの費用対効果の高い管理に対するニーズを背景にしています。ドイツの製薬、バイオテクノロジー、医療機器企業は、法的代理、規制コンサルティング、製品登録などのアウトソーシングサービスへの依存度を高めています。専門性の高いCROの存在と強力な業界標準により、中小企業から大企業まで、多様な適応症と治療領域をカバーするアウトソーシングサービスの導入が促進されています。
アジア太平洋地域の規制関連業務アウトソーシング市場に関する洞察
アジア太平洋地域の薬事アウトソーシング市場は、中国、インド、日本などの国々における製薬、バイオテクノロジー、医療機器産業の急速な拡大に牽引され、2025年から2032年の予測期間中に最も高いCAGRで成長すると見込まれています。医療インフラの拡充、臨床試験を支援する政府の取り組み、そして研究開発活動の活発化により、薬事コンサルティング、臨床試験申請、市販後承認管理といったアウトソーシングサービスの導入が加速しています。中小企業から大企業まで、多くの企業がこれらのサービスを活用し、複数の治療領域における国内外の規制要件へのコンプライアンス確保に努めています。
日本における薬事アウトソーシング市場の洞察
日本の薬事関連業務アウトソーシング市場は、先進的なヘルスケアおよびバイオテクノロジー分野、厳格な規制要件、そして研究開発活動の拡大により、成長を加速させています。企業は、PMDA(医薬品医療機器総合機構)の規制を効果的に遵守するために、製品登録、薬事申請書類作成、臨床試験管理といったサービスをアウトソーシングしています。市場の成長は、腫瘍学、心臓病学、神経学など、様々な適応症における需要に支えられており、中小規模の製薬企業、バイオテクノロジー企業、医療機器企業など、幅広い企業で導入が進んでいます。
中国規制業務アウトソーシング市場に関する洞察
中国の薬事アウトソーシング市場は、2024年にアジア太平洋地域最大の市場収益シェアを占めると予測されています。これは、同国の製薬・バイオテクノロジー産業の拡大、急速な都市化、そして研究開発費の増加によるものです。薬事コンサルティング、臨床試験申請、製品登録、市販後承認管理といったアウトソーシングサービスの需要が高まっており、企業はNMPA規制への効率的なコンプライアンスを実現しています。医薬品、生物製剤、医療機器、ATMP(医薬品・医療機器・医薬品代替医療機器)の分野を問わず、中小企業から大企業まで、製品承認取得と市場参入の迅速化を目指し、薬事アウトソーシングの活用が広がっています。
規制関連業務アウトソーシング市場シェア
規制業務アウトソーシング業界は、主に次のような定評のある企業によって主導されています。
- アクセル・クリニカル・リサーチLLC(米国)
- ジェンパクト(米国)
- CRITERIUM, INC.(米国)
- プロメディカインターナショナル(米国)
- WuXiAppTec(中国)
- メドペース(米国)
- PPD Inc.(米国)
- チャールズリバーラボラトリーズ(米国)
- ICON plc(米国)
- パレクセル・インターナショナル(MA)コーポレーション(米国)
- フレイ(米国)
- ナビタス・クリニカル・リサーチ社(米国)
- メデリス社(米国)
- サイフォーミックス(米国)
- テック・タミナ(米国)
- Acorn Regulatory Consultancy Services Ltd.(アイルランド)
- BIOMAPAS(リトアニア)
- 規制専門家(オーストラリア)
- CompareNetworks, Inc.(米国)
グローバル規制業務アウトソーシング市場の最新動向
- 2025年7月、英国の医薬品・医療製品規制庁(MHRA)は、医薬品および医療機器の承認申請の約40%を信頼できる海外の規制当局に委託すると発表した。この動きは、リソースを節約し、医療における人工知能や先進治療といった革新的な分野に注力することを目的としている。MHRAが2024年4月に導入した「国際承認手続き」により、EU、米国、またはオーストラリアの規制当局の決定に基づく迅速な承認が可能になった。
- 2021年2月、ICON plcは現金と株式による約120億米ドルの取引でPRA Health Sciences, Inc.を買収しました。この買収により、同社のメディカルアフェアーズサービスの提供が強化されました。
- 2021年8月、ProPharma Groupはインドに拠点を置くiSafety Systemsを買収しました。この買収により、ProPharma Groupは、規制およびコンプライアンスコンサルティング、医薬品安全性監視、臨床研究サービス、医療情報サービスにおける世界有数のプロバイダーとしての地位を強化することが期待されます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。