世界の非ホジキンリンパ腫診断市場の規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
12.34 Billion
USD
23.71 Billion
2024
2032
USD
12.34 Billion
USD
23.71 Billion
2024
2032
| 2025 –2032 | |
| USD 12.34 Billion | |
| USD 23.71 Billion | |
|
|
|
|
世界の非ホジキンリンパ腫診断市場セグメンテーション、検査タイプ別(画像診断、生検、免疫組織化学、バイオマーカー、遺伝子検査、細胞遺伝学、腰椎穿刺、血液検査、細胞化学、その他)、がんのステージ別(ステージIV、ステージIII、ステージII、ステージI、ステージ0)、腫瘍の種類別(侵襲性リンパ腫および低悪性度リンパ腫)、製品別(機器ベース製品、プラットフォームベース製品、キットおよび試薬、その他の消耗品)、技術別(蛍光in situハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学、その他)、用途別(スクリーニング、診断および予測、予後、研究)、エンドユーザー別(病院、診断センター、がん研究センター、学術機関、外来手術センター、その他)、流通チャネル別(直接入札、小売)販売、その他) - 2032年までの業界動向と予測
非ホジキンリンパ腫診断市場規模
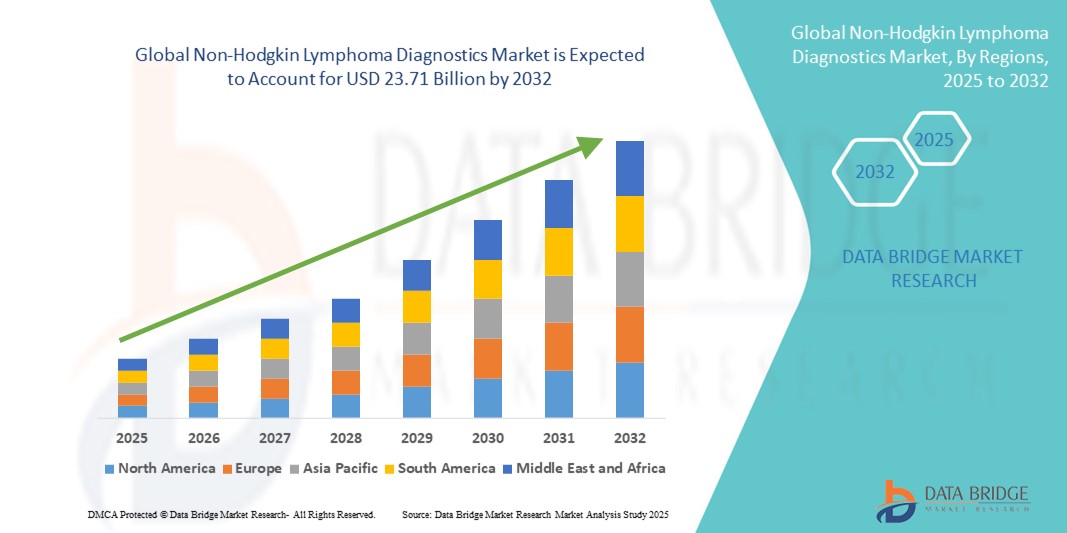
- 世界の非ホジキンリンパ腫診断市場規模は2024年に123.4億米ドルと評価され、予測期間中に8.50%のCAGRで成長し、2032年には237.1億米ドル に達すると予想されています 。
- 市場の成長は主に、世界中で非ホジキンリンパ腫の発生率が増加していることと、がんの早期発見に対する意識が高まっていることによって推進されており、高度な診断技術に対する需要が高まっています。
- さらに、分子診断の拡大、医療費の増加、バイオマーカー検査の継続的な革新により、診断精度とアクセス性が向上しています。これらの動向により、非ホジキンリンパ腫診断の採用が促進され、市場拡大に大きく貢献しています。
非ホジキンリンパ腫診断市場分析
- 非ホジキンリンパ腫(NHL)の診断には、この癌のさまざまなサブタイプを検出および分類するために使用されるさまざまなツールとテクノロジーが含まれており、早期発見、個別化された治療計画、および世界中の医療システム全体での患者転帰の改善に重要な役割を果たしています。
- NHL診断の需要増加は、主にリンパ腫の世界的な罹患率の上昇、分子診断技術の進歩、医療提供者による早期かつ正確な癌検出への重点の増加によって推進されている。
- 北米は、高い認知度、堅牢な医療インフラ、そして次世代シーケンシングやバイオマーカーベースのアッセイなどの革新的な技術がますます採用されている米国における活発な研究イニシアチブに支えられ、2024年には非ホジキンリンパ腫診断市場で42.5%という最大の収益シェアを占めました。
- アジア太平洋地域は、医療へのアクセスの改善、がん検査プログラムの増加、中国やインドなどの国におけるがんの負担の増加により、予測期間中にNHL診断市場で最も急速に成長する地域になると予測されています。
- 免疫組織化学(IHC)セグメントは、リンパ腫のサブタイプの決定に広く使用され、正確な病理診断において重要な役割を果たしているため、2024年には非ホジキンリンパ腫診断市場で39.2%の市場シェアを獲得し、市場を支配しました。
レポートの範囲と非ホジキンリンパ腫診断市場のセグメンテーション
|
属性 |
非ホジキンリンパ腫診断における主要市場洞察 |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
非ホジキンリンパ腫診断市場の動向
「精密医療とAI主導の診断の進歩」
- 非ホジキンリンパ腫(NHL)診断市場における注目すべき進化のトレンドの一つは、精密医療の原理と人工知能(AI)を診断ワークフローに統合することです。これらのイノベーションは、診断精度の向上と個別化された治療戦略の実現を可能にし、臨床医によるNHLの検出、分類、モニタリングの方法を変革しています。
- 例えば、F. ホフマン・ラ・ロシュ社やサーモフィッシャーサイエンティフィック社といった企業は、AIを活用したデジタル病理学プラットフォームや次世代シーケンシング(NGS)ソリューションを提供しており、遺伝子変異やリンパ球細胞のプロファイルをより正確に特定するのに役立ちます。こうしたツールは、60種類以上のNHLサブタイプの鑑別に不可欠であり、患者ごとのケアプランの改善に役立ちます。
- AI駆動型ソフトウェアは、複雑なバイオマーカーや画像データの解析にますます活用されており、臨床医が疾患の進行を示唆する可能性のある形態学的変化を早期に検出するのに役立っています。さらに、AIと統合されたデジタル病理学ソリューションは、特にリソースが限られた環境において、スライド分析の自動化と診断の再現性の向上によりワークフローを効率化できます。
- 液体生検や循環腫瘍DNA(ctDNA)検査も、非侵襲的な診断方法として注目を集めており、疾患の進行や再発をリアルタイムでモニタリングできます。大手診断企業は、これらの新しい技術とAIを組み合わせることで、感度と特異度を高めています。
- 精度、スピード、そして非侵襲性へのこの傾向は、NHL診断のあり方を変革し、より早期の介入と転帰の改善を可能にしています。その結果、イルミナやアジレントテクノロジーズなどの企業は、リアルタイムの疾患追跡をサポートするAI対応のハイスループット診断ツールに投資し、将来を見据えた癌診断における役割を強化しています。
- AI統合型の精密診断ソリューションに対する需要の高まりは、医療システムがリンパ腫の増大する負担を管理するために費用対効果が高く効率的なアプローチを模索する中で、先進国市場と新興国市場の両方で拡大しています。
非ホジキンリンパ腫診断市場の動向
ドライバ
「世界的ながん罹患率の上昇と早期発見の需要」
- 非ホジキンリンパ腫の世界的な発生率の増加と医療提供者による早期癌検出の優先化は、NHL診断市場の成長の重要な原動力となっている。
- 例えば、世界保健機関(WHO)によると、NHLは依然として世界で最も多い癌のトップ10にランクされており、政府や機関は検査の取り組みや診断インフラの強化を迫られている。
- 免疫表現型検査、フローサイトメトリー、分子検査などの診断技術の進歩により、リンパ腫のサブタイプをより正確に特定できるようになり、より早期かつ効果的な治療開始が可能になった。
- さらに、早期診断の重要性に対する患者と医療専門家の意識の高まりと、がん診断に対する保険適用範囲の拡大により、市場での導入がさらに加速しています。
- 病院、がん専門クリニック、診断研究所は、迅速かつ正確な診断に対する高まる需要に応えるために、自動化システムと統合プラットフォームを導入し、積極的に機能をアップグレードしています。
- 個別化された腫瘍学ケアとバイオマーカー主導の意思決定への傾向は、公的および私的医療現場の両方でNHLの高度な診断ツールの採用増加を後押ししている。
抑制/挑戦
「低所得地域における高額な診断費用とアクセスの制限」
- NGS、免疫組織化学、分子プロファイリングなどの高度な診断検査はコストが高いため、特に低・中所得国(LMIC)では、広く普及させる上で大きな課題となっている。
- 例えば、包括的な分子診断に必要なインフラは、機器、試薬、熟練した人員への多額の投資を必要とすることが多く、地方や医療サービスが行き届いていない医療システムでは容易に入手できない可能性があります。
- さらに、訓練を受けた血液病理学者の不足とNHLの正確な診断とサブ分類の複雑さにより、診断の遅れや誤診につながり、タイムリーな治療をさらに複雑にする可能性があります。
- 国際保健機関や官民パートナーシップがこれらのギャップを埋めるために取り組んでいるものの、医療アクセスの格差は依然として市場の成長を妨げている。
- 技術革新による検査コストの削減、がん診断への世界的な資金提供の増加、テレパソロジーやAI支援診断プラットフォームの拡大は、これらの課題の緩和に役立つ可能性があります。より広範な導入には、アクセス性と費用対効果の向上に加え、あらゆる地域で一貫した診断品質を確保することが重要です。
非ホジキンリンパ腫診断市場の展望
市場は、検査の種類、がんのステージ、腫瘍の種類、製品、技術、用途、エンドユーザー、流通チャネルに基づいて分類されています。
- テストの種類別
検査の種類に基づいて、非ホジキンリンパ腫診断市場は、画像診断、生検、免疫組織化学、バイオマーカー、遺伝子検査、細胞遺伝学、腰椎穿刺、血液検査、細胞化学、その他に分類されます。免疫組織化学セグメントは、リンパ腫細胞マーカーの同定と治療計画に不可欠なサブタイプ分類の決定において極めて重要な役割を果たすため、2024年には39.2%という最大の市場収益シェアで市場を席巻しました。診断ラボにおける広範な導入、費用対効果の高さ、そしてホルマリン固定組織サンプルとの適合性から、非ホジキンリンパ腫診断において臨床医の間で好まれる選択肢となっています。
遺伝子検査分野は、個別化医療への関心の高まりと、様々なリンパ腫のサブタイプに特異的な遺伝子変異や転座を特定するニーズの高まりを背景に、2025年から2032年にかけて最も高い成長率を示すと予想されています。次世代シーケンシング(NGS)の進歩と微小残存病変の検出能力の向上は、臨床腫瘍学における遺伝子検査の需要をさらに押し上げています。
- がんのステージ別
がんのステージに基づき、市場はステージIV、ステージIII、ステージII、ステージI、ステージ0に分類されます。ステージIVセグメントは、全身転移に対する徹底的かつ高度な診断評価が必要となる進行期の患者数が多いため、2024年には最大の市場収益シェアを占めました。ステージIV症例における画像診断とバイオマーカープロファイリングの利用増加が、このセグメントの優位性に貢献しています。
ステージ I セグメントは、啓発キャンペーンの拡大、スクリーニング プログラムの強化、先進地域と発展途上地域の両方における診断サービスへのアクセスの拡大による早期発見率の向上により、2025 年から 2032 年にかけて最も速い CAGR を示すことが予想されます。
- 腫瘍の種類別
非ホジキンリンパ腫診断市場は、腫瘍の種類に基づいて、アグレッシブリンパ腫とインドレントリンパ腫に分類されます。びまん性大細胞型B細胞リンパ腫(DLBCL)を含むこれらのサブタイプでは、迅速かつ正確な診断確定が、タイムリーかつ集中的な治療レジメンの開始に必要となるため、アグレッシブリンパ腫セグメントが2024年に最大の市場収益シェアを占め、市場を席巻しました。
低悪性度リンパ腫セグメントは、ゆっくりと進行するリンパ腫では病気の進行やより攻撃的な形態への変化を評価するために継続的な診断モニタリングと追跡検査が必要であるため、2025年から2032年にかけて着実に成長すると予想されています。
- 製品別
製品別に見ると、市場は機器ベース製品、プラットフォームベース製品、キット・試薬、その他消耗品に分類されます。キット・試薬セグメントは、免疫組織化学、フローサイトメトリー、分子検査といった様々な診断手順において繰り返しかつ不可欠な用途で使用されていることから、2024年には最大の市場収益シェアを獲得し、市場を席巻しました。サンプル調製、染色、検出において極めて重要な役割を果たすため、日常的な診断と研究の両方の用途において不可欠な存在となっています。
プラットフォームベース製品セグメントは、分子生物学的検査室における自動化・高スループットプラットフォームの導入増加により、2025年から2032年にかけて最も高い成長率を示すと予想されています。マルチプレックス検査や精密腫瘍学のための統合型で拡張性の高いソリューションへの需要が、病院や診断ラボにおける先進的なプラットフォームの導入を促進しています。
- テクノロジー別
技術に基づいて、非ホジキンリンパ腫診断市場は、蛍光in situハイブリダイゼーション(FISH)、次世代シーケンシング(NGS)、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学、その他に分類されます。免疫組織化学分野は、細胞マーカーの正確な同定とNHLサブタイプの鑑別を可能にすることで、リンパ腫診断の基盤として依然として重要な役割を果たしており、2024年には最大の市場収益シェアを獲得し、市場を席巻しました。病理学研究室での広範な使用とFFPE組織サンプルへの適合性は、その重要性を裏付けています。
次世代シーケンシング(NGS)分野は、包括的なゲノムデータの提供、希少変異の検出、個別化治療戦略のサポートといった能力を背景に、2025年から2032年にかけて最も高い成長率を示すと予想されています。NGSの精度とコスト効率の継続的な向上により、腫瘍学における臨床応用が拡大しています。
- アプリケーション別
用途別に見ると、市場はスクリーニング、診断・予測、予後、研究に分類されます。診断・予測セグメントは、効果的な治療開始における正確な診断とサブタイプ分類の中心的な役割に牽引され、2024年には最大の市場収益シェアを獲得し、市場を席巻しました。予測検査は、標的療法の適格性を判断するのにも役立ち、より良い臨床転帰に貢献します。
個別化医療とリスク層別化への注目が高まるにつれ、病気の進行と治療への反応を予測するバイオマーカーを特定することが必要となり、予後セグメントは2025年から2032年にかけて最も高い成長率を示すことが予想されています。
- エンドユーザー別
エンドユーザーに基づいて、市場は病院、診断センター、がん研究センター、学術機関、外来手術センター、その他に分類されます。病院セグメントは、画像診断、病理検査、分子生物学的検査を含む包括的な診断サービスを提供できるため、2024年には最大の市場収益シェアを獲得し、市場を席巻しました。また、病院はリンパ腫の症状を呈する患者にとって最初の窓口となることが多く、検査件数の増加につながります。
がん研究センター分野は、臨床試験、トランスレーショナルリサーチプログラム、新しい診断ツールや治療ターゲットの探索を目的としたバイオテクノロジー企業との連携の増加に支えられ、2025年から2032年にかけて最も高い成長率を示すことが予想されています。
- 流通チャネル別
流通チャネルに基づいて、市場は直接入札、小売販売、その他に分類されます。直接入札セグメントは、コスト効率とコンプライアンスのために集中調達に依存する政府系病院、医療システム、診断ラボによる大量購入に牽引され、2024年には最大の市場収益シェアを獲得し、市場を席巻しました。
小売販売セグメントは、診断の分散化と家庭用がん検査キットおよびサードパーティのラボサービスに対する消費者の関心の高まりが特に都市市場と先進国でより一般的になるにつれて、2025年から2032年にかけて最も高い成長率を示すことが予想されています。
非ホジキンリンパ腫診断市場の地域分析
- 北米は、高い認知度、堅牢な医療インフラ、そして次世代シーケンシングやバイオマーカーベースのアッセイなどの革新的な技術がますます採用されている米国における活発な研究イニシアチブに支えられ、2024年には非ホジキンリンパ腫診断市場を42.5%という最大の収益シェアでリードしました。
- この地域の患者と医療提供者は、免疫組織化学、フローサイトメトリー、次世代シーケンシングなどのツールを活用して正確なサブタイプ分類と治療計画を行い、精密診断を優先しています。
- この強力な市場ポジションは、多額の医療費支出、がんの早期発見に対する意識の高まり、進行中の研究イニシアチブによってさらに支えられており、北米は病院、がんセンター、学術機関におけるリンパ腫診断の革新と採用の重要な拠点として位置付けられています。
米国における非ホジキンリンパ腫診断市場の洞察
米国の非ホジキンリンパ腫診断市場は、2024年には北米最大の収益シェア(79.3%)を獲得しました。これは、米国におけるリンパ腫の有病率の高さと高度な診断インフラの整備が牽引しています。大手バイオテクノロジー企業および診断企業の存在、そしてがん研究への積極的な投資が、診断プラットフォームの革新を促進しています。がんの早期発見に対する意識の高まりと、分子生物学的技術およびイメージング技術への幅広いアクセスが、市場の継続的な成長を支えています。さらに、米国の医療制度はプレシジョン・メディシン(精密医療)と個別化治療を重視しており、正確な診断ツールへの需要が高まっています。
欧州における非ホジキンリンパ腫診断市場の洞察
欧州における非ホジキンリンパ腫診断市場は、主にがんスクリーニングプログラムの増加と人口の高齢化を背景に、予測期間中に大幅な年平均成長率(CAGR)で成長すると予測されています。この地域では、標準化された診断プロトコルと早期発見が重視されており、フローサイトメトリーや免疫組織学的検査(IHC)といった先進技術への需要が高まっています。がん研究と医療のデジタル化に対する政府の支援の拡大、そして啓発活動の拡大により、この地域の病院や検査室におけるNHL診断の導入が拡大しています。
英国における非ホジキンリンパ腫診断市場の洞察
英国の非ホジキンリンパ腫診断市場は、強力な公的医療制度とがん診断の改善に向けた積極的な政府の取り組みにより、予測期間中に堅調な年平均成長率(CAGR)で成長すると予想されています。バイオマーカー研究への投資の増加と、早期かつ正確な検出法を求める患者の関心の高まりが、市場拡大をさらに促進しています。さらに、学術機関と診断企業との戦略的提携により、新たな検査技術の開発が促進されています。
ドイツにおける非ホジキンリンパ腫診断市場の洞察
ドイツの非ホジキンリンパ腫診断市場は、高度に発達した医療インフラと高度な検査能力に支えられ、予測期間を通じて大幅な成長が見込まれています。同国は精密診断に注力し、臨床研究と個別化医療にも力を入れており、ゲノム検査や分子生物学的検査の導入を加速させています。さらに、ドイツの医療保険償還政策と技術革新は、公的および民間の診断施設における市場浸透を促進しています。
アジア太平洋地域の非ホジキンリンパ腫診断市場に関する洞察
アジア太平洋地域の非ホジキンリンパ腫診断市場は、がん罹患率の上昇、医療アクセスの向上、診断インフラの拡充を背景に、2025年から2032年の予測期間中に23.6%という最も高いCAGRで成長すると予測されています。中国、日本、インドなどの国々では、政府主導の保健政策や腫瘍学への投資増加により、高度な診断技術の導入が進んでいます。この地域ではデジタル診断や分子診断の導入が進んでおり、市場は都市部および準都市部で急速に拡大しています。
日本における非ホジキンリンパ腫診断市場の洞察
日本の非ホジキンリンパ腫診断市場は、高齢化と早期発見への強い関心により、成長を続けています。日本の医療制度は質の高い診断を重視しており、免疫組織化学や次世代シークエンシングといった技術の導入率も高くなっています。さらに、AIを活用した診断ツールの導入や予防医療の文化も、病院や腫瘍センターにおける信頼性の高いNHL診断ソリューションの需要を高めています。
インドにおける非ホジキンリンパ腫診断市場の洞察
インドの非ホジキンリンパ腫診断市場は、医療インフラの発展、患者数の増加、そしてがん診断に対する認知度の高まりに支えられ、2024年にはアジア太平洋地域最大の市場収益シェアを占めました。急速な都市化、診断検査の低価格化、そして政府支援によるスクリーニングプログラムが、大都市圏および第二級都市における需要を牽引しています。地域におけるイノベーションと民間診断ラボの増加も、市場の上昇傾向に寄与しています。
非ホジキンリンパ腫診断市場シェア
非ホジキンリンパ腫診断業界は、主に、以下を含む定評のある企業によって牽引されています。
- キヤノンメディカルシステムズ株式会社(日本)
- Koninklijke Philips NV (オランダ)
- シーメンス ヘルスケア AG(ドイツ)
- ダナハーコーポレーション(米国)
- バイオ・ラッド・ラボラトリーズ社(米国)
- ゼネラル・エレクトリック・カンパニー(米国)
- シスメックス株式会社(日本)
- グレイル(米国)
- F. ホフマン・ラ・ロシュ社(スイス)
- 東軟集団(中国)
- アジレント・テクノロジーズ社(米国)
- ネオジェノミクス・ラボラトリーズ(米国)
- ホロジック社(米国)
- インテグレーテッドDNAテクノロジーズ社(米国)
- CENTOGENE NV(ドイツ)
- メリットメディカルシステムズ(米国)
- ラボコープ・ジェネティクス社(米国)
- パーキンエルマー(米国)
- キアゲン(米国)
- GeneDx LLC(米国)
世界の非ホジキンリンパ腫診断市場の最近の動向は何ですか?
- 2023年4月、ロシュ・ダイアグノスティックスは、非ホジキンリンパ腫(NHL)を含む造血悪性腫瘍に特化した新たなコンパニオン診断アッセイを導入し、免疫組織化学(IHC)ポートフォリオを拡充すると発表しました。このイノベーションは、診断精度と治療選択の向上を目指しており、ロシュの腫瘍診断におけるリーダーシップを強化し、がん治療における個別化医療の高まる需要を支えるものです。
- サーモフィッシャーサイエンティフィックは2023年3月、非ホジキンリンパ腫のサブタイプの正確な同定と分類に特化した、先進的なマルチプレックスフローサイトメトリーパネルを発売しました。この新しい診断ソリューションは、複雑な血液悪性腫瘍に関するより深い知見を提供しながら、検査室の効率性を向上させるものであり、サーモフィッシャーサイエンティフィックの精密診断と血液病理学研究の発展への取り組みを改めて強調するものです。
- 2023年3月、アジレント・テクノロジーズは、NHLの早期発見のための分子診断アッセイの開発と検証を加速するため、世界的ながん研究コンソーシアムと協力契約を締結しました。この取り組みは、アジレントのハイスループットゲノミクスプラットフォームと実臨床データを統合し、標的診断による疾患予後の改善とタイムリーな治療介入を可能にすることを目指しています。
- 2023年2月、バイオ・ラッド・ラボラトリーズ社は、非ホジキンリンパ腫患者における微小残存病変(MRD)の検出を目的とした次世代デジタルPCRアッセイを発表しました。この技術は、早期再発予測と個別化治療モニタリングをサポートし、血液腫瘍学における疾患管理と治療後サーベイランスに大きな進歩をもたらします。
- 2023年1月、アボット・ラボラトリーズは、アジア太平洋地域の主要な腫瘍センターとの戦略的提携を発表しました。これは、特にリソースが限られている環境において、リンパ腫診断プラットフォームへのアクセスを拡大するためのものです。この提携は、新興市場における免疫測定および分子検査能力の拡大に焦点を当てており、アボットの世界的な健康の公平性と血液がんの早期発見へのコミットメントを示しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。