世界の分子ポイントオブケア検査(NAAT使用)市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
37.93 Billion
USD
86.17 Billion
2024
2032
USD
37.93 Billion
USD
86.17 Billion
2024
2032
| 2025 –2032 | |
| USD 37.93 Billion | |
| USD 86.17 Billion | |
|
|
|
|
世界の分子ポイントオブケア検査(NAAT使用)市場セグメンテーション、製品(機器、消耗品、試薬)、適応症(呼吸器感染症検査、性感染症(STI)検査、消化管感染症検査など)、エンドユーザー(検査室、病院、診療所、外来センター、在宅ケア、介護施設など)、検査モード(処方箋に基づく検査およびOTC検査)、流通チャネル(病院薬局、小売薬局、オンライン薬局) - 2032年までの業界動向と予測
分子ポイントオブケア検査(NAAT使用)市場規模
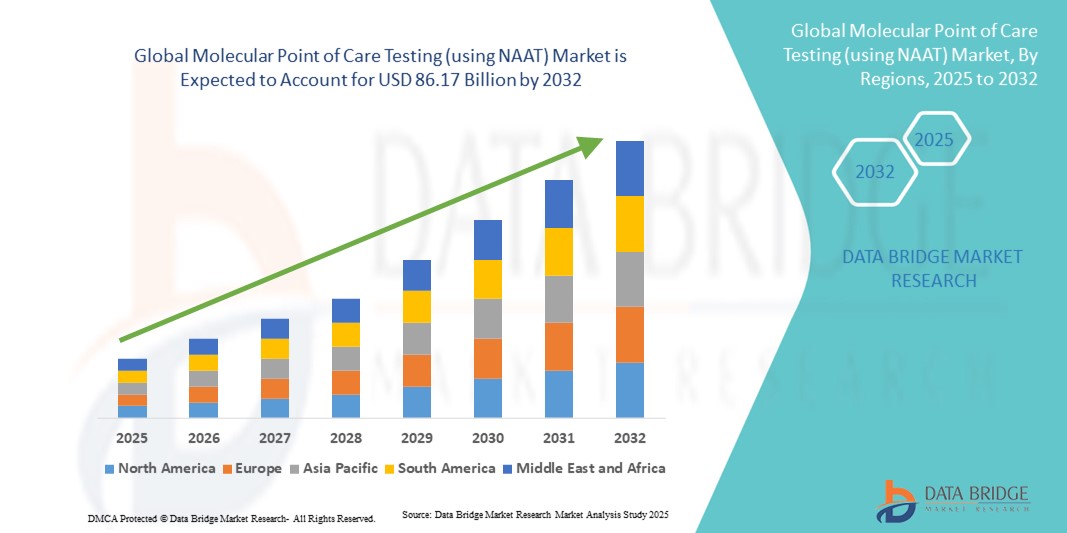
- 世界の分子ポイントオブケア検査(NAATを使用)市場規模は2024年に379.3億米ドルと評価され、予測期間中に1.08%のCAGRで成長し、2032年までに861.7億米ドル に達すると予想されています 。
- この市場の成長は、感染症の蔓延の増加、迅速かつ正確な診断検査の需要、分子診断技術の進歩によって主に推進されています。
- さらに、分散型医療への移行とマルチプレックス検査の導入拡大が、分子POCT市場の拡大に貢献しています。これらの要因により、分子POCTは現代の診断における極めて重要な要素として位置づけられ、様々な健康状態をタイムリーかつ正確に検出することが可能となっています。
分子ポイントオブケア検査(NAAT使用)市場分析
- 核酸増幅検査(NAAT)を用いた分子ポイントオブケア検査(POCT)は、患者の現場またはその近くで感染症やその他の病状を迅速かつ正確に検出できるため、病院、診療所、分散型医療環境における現代の医療診断の重要な要素となっています。
- 分子POCTの採用増加の主な要因は、感染症の蔓延の増加、タイムリーで正確な診断の需要の高まり、そして携帯型で使いやすい分子検査機器の技術的進歩である。
- 北米は、高度な診断技術の早期導入、高い医療費、主要な業界プレーヤーの強力な存在に支えられ、2024年には分子POCT市場で39%という最大の収益シェアを獲得して市場を支配しました。米国では、病院、診療所、救急医療現場でのNAATベースの検査の統合により、大幅な成長が見込まれています。
- アジア太平洋地域は、医療インフラ投資の増加、迅速診断ソリューションの認知度向上、新興経済国におけるポイントオブケア検査の需要増加により、予測期間中に最も急速に成長する地域になると予想されています。
- 呼吸器感染症検査セグメントは、感染性呼吸器疾患の有病率の高さと、タイムリーな治療と封じ込め対策を導くための迅速かつ正確な診断の臨床ニーズにより、2024年には分子POCT市場を支配し、43.2%の市場シェアを獲得しました。
レポートの範囲と分子ポイントオブケア検査(NAATを使用)市場セグメンテーション
|
属性 |
分子ポイントオブケア検査(NAAT使用)主要市場インサイト |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
分子ポイントオブケア検査(NAAT使用)市場動向
迅速、多重、AIを活用した検査の進歩
- 世界の分子POCT市場における重要かつ加速的なトレンドは、迅速で多重化されたNAATデバイスとAI対応診断プラットフォームの開発であり、病院、診療所、分散型医療環境全体でのポイントオブケア検査の速度、精度、および使いやすさを向上させています。
- 例えば、Xpert Xpress SARS-CoV-2/Flu/RSV検査は、1回の実行で複数の病原体検出を統合し、ターンアラウンドタイムを短縮し、患者現場でのワークフローを合理化します。
- 分子POCTへのAI統合により、検査結果の自動解釈、疾病発生の予測分析、インテリジェントな品質管理などの機能が可能になり、診断の信頼性が向上し、人的ミスが削減されます。
- これらのAI駆動型プラットフォームにより、医療提供者は複数の診断パラメータを同時に管理し、重篤な症状に対してより迅速な結果を提供し、集中化された検査室への依存を減らすことができる。
- より迅速で正確、かつ相互接続された検査ソリューションへのこの傾向は、POC診断への期待を再構築しています。その結果、アボットやロシュなどの企業は、マルチプレックス機能と臨床スタッフ向けのユーザーフレンドリーなインターフェースを備えたAI対応NAATデバイスを開発しています。
- 医療提供者が迅速かつ信頼性の高い診断を優先する傾向が強まるにつれ、マルチプレックス検出とAI駆動機能を備えた分子POCTデバイスの需要は、病院と外来の両方の環境で急速に高まっています。
分子ポイントオブケア検査(NAAT使用)市場動向
ドライバ
感染症の負担と分散検査による需要の高まり
- 世界的な感染症の蔓延の増加と分散型医療サービスへの移行は、NAATを使用した分子POCTの採用拡大の大きな原動力となっている。
- 例えば、2024年にセフェイドは、性感染症や呼吸器パネルを含むGeneXpertプラットフォームの拡張アプリケーションを開始し、ポイントオブケアでの診断ニーズの高まりに対応するための取り組みを反映しています。
- 医療提供者がより迅速な診断とタイムリーな治療を目指す中、NAATベースのPOCTは高い感度、特異性、迅速な結果を提供し、従来の集中検査室検査に代わる魅力的な選択肢を提供します。
- さらに、アウトブレイク管理と緊急事態への備えの重要性が高まっているため、分子POCTは感染症管理プログラムの不可欠な要素となり、迅速な隔離と治療の決定を可能にしています。
- 患者に近い場所での検査の利便性、最小限のサンプル処理要件、および複数の検査を同時に管理する能力は、病院、診療所、および現場でのNAATベースの分子POCTの採用を推進する重要な要因です。
抑制/挑戦
規制上のハードルと運用上の制限
- 分子POCT機器の規制遵守と厳格な承認プロセスは、NAATベースのプラットフォームが商業化される前に精度、安全性、信頼性に関する厳格な基準を満たす必要があるため、市場拡大に大きな課題をもたらします。
- 例えば、FDAやCEの承認の遅れは革新的なPOCT製品の発売を遅らせ、時間的に制約のある状況での高度な診断へのアクセスを制限する可能性がある。
- NAATデバイスの技術的な利点にもかかわらず、訓練を受けた人員、デバイスのメンテナンス、消耗品の供給などの運用上の制限により、リソースが限られた環境での導入が制限される可能性があります。
- さらに、従来の迅速検査と比較して、高度なマルチプレックスまたはAI対応のPOCTデバイスは比較的高価であるため、小規模なクリニックや低所得地域では障壁となり、広範な導入が制限される可能性があります。
- コストは徐々に低下しているものの、高度な分子POCTプラットフォームに対する認識されたプレミアムは、予算に敏感な医療現場での導入を妨げる可能性がある。
- 合理化された規制経路、スタッフのトレーニング、費用対効果の高いデバイスの開発を通じてこれらの課題を克服することは、持続的な市場成長にとって不可欠です。
分子ポイントオブケア検査(NAAT使用)市場の範囲
市場は、製品、適応症、エンドユーザー、テストモード、流通チャネルに基づいてセグメント化されています。
- 製品別
製品に基づいて、分子POCT市場は、機器と消耗品・試薬に分類されます。機器セグメントは、迅速かつ正確な結果を提供する上での自動NAATデバイスの重要な役割に牽引され、2024年には55.3%という最大の収益シェアで市場を支配しました。医療施設は、高いスループット、信頼性、および検査情報システムとの統合性から、機器の導入を優先しています。最新の機器への投資により、病院や検査室はワークフローを合理化し、効率を向上させ、感染症診断のターンアラウンドタイムを短縮できます。このセグメントは、分散テストに適したポータブルでコンパクトな機器などの技術革新の恩恵を受けています。機器は、自動レポート作成と品質管理を容易にするために、ソフトウェアプラットフォームとペアになっていることがよくあります。メーカーが提供する継続的な技術サポートとメンテナンスサービスも、導入をさらに後押ししています。
消耗品・試薬セグメントは、NAATベースのPOCTで使用されるカートリッジ、検査キット、試薬の継続的な需要に支えられ、2025年から2032年にかけて12.5%という最も高いCAGRを達成すると予想されています。消耗品は、病院、診療所、検査室における日常的な検査業務に不可欠であり、サプライヤーに継続的な収益をもたらします。感染症検査に対する意識の高まりと検査頻度の増加が需要を押し上げています。特殊な試薬を必要とするマルチプレックスアッセイは、成長をさらに加速させます。試薬の安定性と使いやすさを向上させる技術革新も、普及に貢献しています。また、このセグメントは、配送時間とコストを削減するサプライチェーンの改善からも恩恵を受けています。
- 適応症別
適応症に基づいて、市場は呼吸器感染症検査、性感染症(STI)検査、消化管感染症検査、その他に分類されています。SARS-CoV-2、インフルエンザ、RSウイルスなどの病原体の蔓延率が高いため、呼吸器感染症検査セグメントは2024年に43.2%のシェアで市場を支配しました。呼吸器感染症を迅速かつ正確に検出することは、病院、診療所、救急医療ユニットにおいて、タイムリーな治療と封じ込めのために不可欠です。NAATベースのPOCTは高い感度と特異性を提供するため、呼吸器感染症の好ましい方法となっています。政府のアウトブレイク監視および制御プログラムによって、採用がさらに促進されています。病院や検査室では、迅速な意思決定のためにこれらの検査を救急医療のワークフローに統合しています。マルチプレックスパネルの継続的なイノベーションにより、スループットと効率が向上します。
性感染症検査分野は、性感染症に対する意識の高まり、スクリーニングプログラムの普及、そして発生率の上昇に牽引され、2025年から2032年にかけて13.1%という最も高いCAGRを達成すると予想されています。NAATベースのPOCTは、迅速、機密性、そして正確な性感染症の検出を可能にし、クリニックや自宅での検査に最適です。1回の検査で複数の性感染症を検出するマルチプレックス検査の普及が、その普及を加速させています。自己検査や性健康に関する取り組みの拡大も、需要を押し上げています。遠隔医療プラットフォームとの統合により、結果報告とカウンセリングの質が向上します。診断会社と医療提供者間のパートナーシップの増加も、市場の成長をさらに促進します。
- エンドユーザー別
分子POCT市場は、エンドユーザーに基づいて、検査室、病院、診療所、外来センター、在宅ケア、高齢者施設、その他に分類されます。病院セグメントは、患者数の増加と迅速な診断ニーズにより、2024年には48.6%という最大の収益シェアで市場を席巻しました。病院は、ワークフローの改善、感染症の発生管理、救急医療の支援を目的として、高度なNAAT装置に投資しています。病院情報システムとの統合により、業務効率が向上します。このセグメントは、継続的な技術革新と政府の優遇措置の恩恵を受けています。病院では、複数の検査タイプに対応できる機器が必要になる場合が多く、サプライヤーとの長期契約により、機器と消耗品の安定した供給が確保されています。
ホームケア分野は、在宅検査ソリューション、利便性、プライバシーへの需要の高まりを背景に、2025年から2032年にかけて14.2%という最も高いCAGR(年平均成長率)を達成すると予想されています。ホームケア用NAATキットを使用すれば、患者はクリニックや病院に行くことなく検査を受けることができます。呼吸器感染症や性感染症の自己検査が普及を促進しています。スマートフォンアプリとの連携により、結果の解釈や遠隔医療レポート作成が容易になります。啓発キャンペーンや遠隔医療提供者との提携により、リーチが拡大しています。保健当局による在宅検査の受け入れ拡大も、持続的な成長を支えています。
- テストモード別
検査方法に基づいて、市場は処方箋に基づく検査と市販薬(OTC)による検査に分類されます。処方箋に基づく検査セグメントは、規制要件と専門家の監督に牽引され、2024年には62.3%のシェアで市場を支配しました。処方箋に基づく検査は、特に病院や診療所において、正確性、信頼性、そして臨床的解釈を提供します。医療ワークフローとの統合により、患者の安全と品質保証が確保されます。専門家の指導が必要な高リスク感染症では、導入が進んでいます。検査機器は、結果の追跡と報告のために病院ネットワークに接続されることがよくあります。メーカーは、規制基準を満たしつつ、使いやすさの向上に取り組んでいます。
OTC検査セグメントは、呼吸器感染症、性感染症、胃腸感染症の自己検査需要の高まりを背景に、2025年から2032年にかけて13.5%という最も高いCAGR(年平均成長率)を達成すると予想されています。使いやすいキットとスマートフォン対応の解析ツールにより、検査へのアクセス性が向上します。消費者は、利便性、プライバシー保護、そして通院回数の削減という理由から、OTCソリューションを好んでいます。啓発活動や遠隔医療の導入が普及を促進しています。小売業者やオンライン薬局は、検査キットの入手性を向上させています。このセグメントは、家庭用に設計された簡素化されたパッケージ済みの検査キットの恩恵を受けています。
- 流通チャネル別
流通チャネルに基づいて、市場は病院薬局、小売薬局、オンライン薬局に分類されます。病院薬局セグメントは、入院患者および外来患者向けの検査の直接調達に牽引され、2024年には57.4%のシェアで市場を支配しました。病院は、NAAT機器、消耗品、試薬の安定供給のために薬局チャネルを活用しています。一括購入契約はコストを削減し、タイムリーな補充を保証します。病院のサプライチェーンシステムとの統合は、在庫切れを最小限に抑えます。病院は薬局契約を通じて技術サポートやトレーニングを受けることがよくあります。メーカーとの提携は、製品の入手可能性とサービスを強化します。
オンライン薬局セグメントは、2025年から2032年にかけて、利便性の高い注文方法、NAATキットの宅配、そして遠隔医療の普及拡大を背景に、15.1%という最も高いCAGRを達成すると予想されています。オンラインプラットフォームは、処方薬と市販薬の両方の検査を目立たずに購入することを可能にします。デジタルマーケティングキャンペーンは消費者の認知度を高め、遠隔医療アプリとの連携により、検査結果をシームレスに報告できます。消費者は利便性とプライバシーを重視し、オンラインアクセスを好む傾向が高まっています。Eコマースの成長と物流の改善は、この流通チャネルの急速な拡大を支えています。
分子ポイントオブケア検査(NAAT使用)市場地域分析
- 北米は、高度な診断技術の早期導入、高い医療費、主要な業界プレーヤーの強力な存在に支えられ、2024年には分子POCT市場で39%という最大の収益シェアを獲得して市場を支配しました。米国では、病院、診療所、救急医療現場でのNAATベースの検査の統合により、大幅な成長が見込まれています。
- この地域の医療提供者は、迅速で正確かつ信頼性の高い検査を重視しており、NAATベースのPOCTデバイスは病院、診療所、救急医療施設にとって不可欠なものとなっています。特に米国では、既存の診断企業とスタートアップ企業の双方によるイノベーションに支えられ、NAATデバイスが病院、外来診療所、在宅医療検査に導入され、大幅な成長を遂げています。
- 強力な医療インフラ、高い医療費、そして技術的に進歩した人口によって、この広範な導入はさらに支えられている。
米国における分子ポイントオブケア検査(NAAT使用)市場インサイト
米国の分子POCT市場は、高度な診断技術の急速な導入と、感染症の迅速な検出ニーズの高まりを背景に、2024年には北米最大の収益シェア(79%)を獲得しました。医療提供者は、呼吸器感染症、性感染症、胃腸感染症の検査において、その高い精度と迅速な結果から、NAATベースの検査をますます重視しています。分散型医療と在宅検査の台頭も市場をさらに牽引しています。さらに、分子POCTデバイスと病院情報システムおよび遠隔医療プラットフォームの統合も、市場の拡大に大きく貢献しています。主要な診断企業の強力なプレゼンスと継続的な技術革新も、持続的な成長を支えています。
欧州における分子ポイントオブケア検査(NAAT使用)市場インサイト
欧州の分子POCT市場は、主に迅速診断の需要増加と感染症発生率の上昇を背景に、予測期間を通じて大幅なCAGRで拡大すると予測されています。ポイントオブケア検査と医療のデジタル化を推進する政府の取り組みは、病院、診療所、検査室における導入を促進しています。この成長は、都市化と多剤耐性感染症の蔓延増加にも支えられています。欧州の医療機関では、タイムリーな疾患管理とアウトブレイク抑制のため、NAATベースの検査の導入が進んでいます。在宅医療施設と商業医療施設の両方で、患者ケアの向上を目的としてPOCT機器が導入されています。市場はドイツ、フランス、イタリアなどの国で著しい成長を遂げています。
英国における分子ポイントオブケア検査(NAAT使用)市場インサイト
英国の分子POCT市場は、迅速診断と分散型検査ソリューションの需要の高まりを背景に、予測期間中に注目すべきCAGRで成長すると予想されています。NAATベースの検査の利点(高い感度と特異度など)に対する医療提供者の認識の高まりは、病院や診療所での導入を促進しています。感染症の早期発見と管理に向けた政府の取り組みも、市場拡大をさらに後押ししています。英国の強力な医療インフラと堅牢なeヘルスシステムは、デバイスの統合と結果報告を強化しています。公的医療部門と民間医療部門の両方で導入が拡大していることが、市場の成長を刺激しています。遠隔医療と在宅ケア検査の取り組みは、英国における導入をさらに促進すると予想されます。
ドイツにおける分子ポイントオブケア検査(NAAT使用)市場インサイト
ドイツの分子POCT市場は、感染症の蔓延と迅速診断の需要増加に支えられ、予測期間中に大幅なCAGRで拡大すると予想されています。ドイツの高度な医療インフラ、技術革新への強いこだわり、そして医療従事者の高い認知度は、NAATベースのPOCTの導入を促進しています。POCT機器を病院情報システムや検査ネットワークと統合することで、業務効率が向上します。外来診療所や外来センターでは、分散型検査への関心が高まっており、在宅医療サービスと商業診断施設の両方で導入が増加しています。POCT機器および消耗品の継続的な研究開発と現地生産は、市場の成長をさらに加速させます。
アジア太平洋地域における分子ポイントオブケア検査(NAAT使用)市場インサイト
アジア太平洋地域の分子POCT市場は、2025年から2032年の予測期間中、感染症の発生率上昇、医療投資の増加、そして中国、日本、インドなどの国々における検査インフラの拡大を背景に、23.5%という最も高いCAGRで成長すると見込まれています。この地域では、分散型および在宅ベースの検査ソリューションへの関心が高まっており、その導入が加速しています。デジタルヘルスケアと感染症モニタリングを促進する政府プログラムも市場拡大を支えています。現地メーカーの存在は、NAAT機器と消耗品の低価格化を保証しています。都市化の進展、技術導入、そして早期診断への意識の高まりも、成長をさらに加速させています。病院、診療所、在宅ケアの現場における需要は着実に増加しています。
日本における分子ポイントオブケア検査(NAAT使用)市場インサイト
日本の分子POCT市場は、高い医療水準、高度な診断技術の導入、そして迅速検査への意識の高まりにより、勢いを増しています。日本では感染症の正確かつ迅速な検出を重視しており、病院、診療所、在宅医療の現場での導入が進んでいます。NAAT機器を病院ネットワークや遠隔医療プラットフォームと統合することで、効率性が向上します。高齢化社会の進展により、ユーザーフレンドリーでアクセスしやすい検査ソリューションへの需要がさらに高まっています。マルチプレックス化とポータブル化が進むNAAT機器の継続的なイノベーションが市場を支えています。予防医療とデジタル診断への関心の高まりは、在宅医療と商業医療の両方の分野で導入を加速させています。
インドにおける分子ポイントオブケア検査(NAAT使用)市場インサイト
インドの分子POCT市場は、感染症の蔓延率の高さ、中流階級の拡大、そしてヘルスケア意識の高まりを背景に、2024年にアジア太平洋地域最大の市場収益シェアを占めました。急速な都市化と、スマートヘルスケアおよびデジタル診断に向けた政府の取り組みが、主要な成長ドライバーとなっています。病院、診療所、在宅ケアサービスでは、迅速な診断と治療のためにNAATベースのPOCT機器の導入が進んでいます。地元メーカーの手頃な価格の機器は、都市部および準都市部におけるアクセス性を向上させています。マルチプレックス検査ソリューションへの需要の高まりも、この導入をさらに加速させています。強力な公衆衛生プログラムと診断企業との提携が、引き続き市場の成長を牽引しています。
分子ポイントオブケア検査(NAAT使用)市場シェア
分子ポイントオブケア検査(NAAT を使用)業界は、主に次のような定評のある企業によって主導されています。
- サーモフィッシャーサイエンティフィック社(米国)
- ホロジック社(米国)
- BD(米国)
- F. ホフマン・ラ・ロシュ社(スイス)
- アボット(米国)
- QIAGEN(オランダ)
- BIOMÉRIEUX(フランス)
- ダナハー(米国)
- イルミナ社(米国)
- シスメックス株式会社(日本)
- シーメンス・ヘルシニアーズAG(ドイツ)
- シージェン株式会社(韓国)
- ガーダント・ヘルス社(米国)
- ラボコープ(米国)
- Exact Sciences Corporation(米国)
- 10x Genomics, Inc.(米国)
- DNAジェノテック社(カナダ)
- PathoNostics(オランダ)
- モルビオ・ダイアグノスティクス・リミテッド(インド)
世界の分子ポイントオブケア検査(NAAT を使用)市場の最近の動向は何ですか?
- 2025年7月、BD(ベクトン・ディッキンソン・アンド・カンパニー)は、SARS-CoV-2用のBD Veritor™システムについてFDA 510(k)認可を取得しました。このデジタル検査は、医師の診療所、救急診療所、小売店などの診療所で、症状のある人のCOVID-19抗原を約15分で検出するように設計されているものです。
- 2024年10月、世界保健機関(WHO)は、MPOX(旧称サル痘)の緊急使用を目的とした初の診断検査を承認しました。この承認は、特に資源が限られた地域において、MPOXの迅速かつ正確な診断への世界的なアクセスを向上させることを目的としています。この検査は核酸増幅技術を用いており、高い感度と特異性を備えたポイントオブケア検査を可能にします。
- ロシュ・ダイアグノスティックスは2024年4月、検査室の生産性向上とエラー削減を目的とした次世代分子検査自動化プラットフォーム「cobas® 5800システム」を発表しました。このシステムは標準化されたアッセイと拡張可能なソリューションを提供し、様々な検査量や組み合わせに対応します。cobas® 5800システムは、分子診断における自動化を強化することで、ワークフローを合理化し、検査環境全体で一貫した結果を確保することを目指しています。
- 2023年3月、QuidelOrtho Corporationは、米国食品医薬品局(FDA)からDe Novo申請が承認され、同社の新製品であるSofia® 2 SARS Antigen+ FIAの販売が許可されたと発表しました。Sofia 2 SARS Antigen+ FIAは、COVID-19を検出する迅速抗原検査としてFDAの承認を取得した初の製品です。
- 2023年3月、LumiraDx SARS-CoV-2抗原検査は、CLIA免除証明書、コンプライアンス証明書、または認定証明書に基づいて運営されている患者ケア施設におけるポイントオブケアでの使用が承認されました。この検査は、ポイントオブケア環境での検査実施に熟練した医療専門家または検査技師による使用を目的としています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。