世界の遺伝性癌検査市場、検査タイプ別(マルチパネルセット、単一部位遺伝子検査)、診断タイプ別(生検、画像診断、臨床検査)、技術別(シーケンシング、ポリメラーゼ連鎖反応(PCR)、マイクロアレイ)、疾患タイプ別(遺伝性乳癌・卵巣癌症候群、コーデン症候群、リンチ症候群、遺伝性白血病・造血悪性腫瘍症候群、家族性大腸腺腫症(FAP)、リ・フラウメニ症候群、フォン・ヒッペル・リンドウ病、多発性内分泌腫瘍(MEN)症候群)、エンドユーザー別(病院、診療所、研究所、放射線科センター、診断センター、その他)、流通チャネル別(直接入札、小売販売)、業界動向および2029年までの予測。
遺伝性癌検査市場 市場分析と洞察
がんは、細胞の機能を制御する遺伝子の特定の変異によって引き起こされる遺伝病であり、特に細胞の増殖と再生に影響を及ぼします。遺伝性の遺伝子変異は、すべてのがんの約5〜10%の原因です。研究者は、特定の遺伝子の変異を、特定のがんの発症を通じて人々に影響を与える50を超える遺伝性がん症候群に関連付けています。さらに、乳がん症例の約5〜10%は、親から受け継いだ遺伝子変異に関連しています。したがって、がんの有病率の増加は遺伝性がんの着実な成長を促進し、したがって遺伝性がん検査市場の成長を促進しています。さらに、非侵襲性検査方法の需要の増加と、より質の高いヘルスケアと早期診断の需要の高まりは、市場成長の大きな機会です。さらに、遺伝性がん検査中に直面する倫理的課題と市場プレーヤー間の競争の激化は、市場成長の重要な課題です。
しかし、がん診断に関する厳格な規制と検査に伴う高額な費用が市場の成長を妨げる可能性があります。
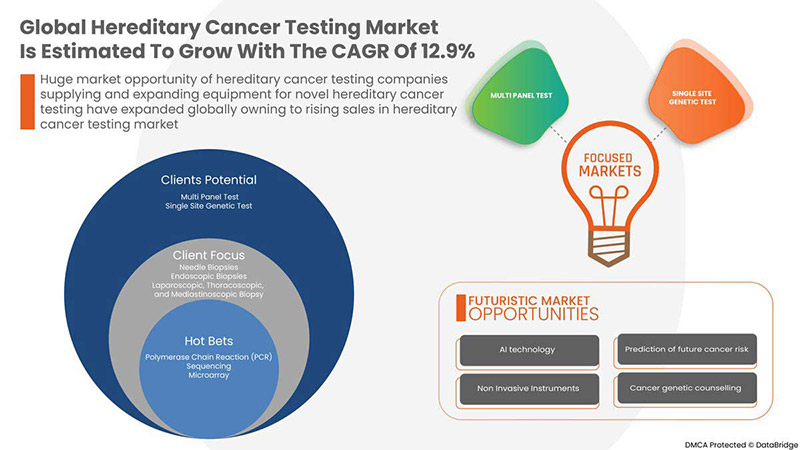
Data Bridge Market Research は、世界の遺伝性癌検査市場は、予測期間中に 12.9% の CAGR で成長し、2029 年までに 130 億 8,504 万米ドルに達すると予測しています。この市場レポートでは、価格分析、特許分析、技術進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 (2019 - 2015 にカスタマイズ可能) |
|
定量単位 |
売上高(百万米ドル)、販売数量(個数)、価格(米ドル) |
|
対象セグメント |
検査タイプ(マルチパネルセット、単一部位遺伝子検査)、診断タイプ(生検、画像診断、臨床検査)、技術(シーケンシング、ポリメラーゼ連鎖反応(PCR)、マイクロアレイ)、疾患タイプ(遺伝性乳がん・卵巣がん症候群、コーデン症候群、リンチ症候群、遺伝性白血病・造血悪性腫瘍症候群、家族性大腸腺腫症(FAP)、リ・フラウメニ症候群、フォン・ヒッペル・リンドウ病、多発性内分泌腫瘍症(MEN)症候群)、エンドユーザー(病院、クリニック、研究所、放射線科センター、診断センター、その他)、流通チャネル(直接入札、小売販売)別。 |
|
対象国 |
米国、カナダ、メキシコ、ドイツ、フランス、英国、イタリア、スペイン、ロシア、トルコ、ベルギー、オランダ、スイス、その他のヨーロッパ諸国、中国、日本、インド、韓国、シンガポール、タイ、マレーシア、オーストラリア、フィリピン、インドネシア、その他のアジア太平洋諸国、南アフリカ、サウジアラビア、UAE、エジプト、イスラエル、その他の中東およびアフリカ諸国、ブラジル、アルゼンチン、その他の南米諸国。 |
|
対象となる市場プレーヤー |
Invitae Corporation、Illumina, Inc.、Natera, Inc.、CENTOGENE NV、4baseCare、Biocartis、Fulgent Genetics、Ambry Genetics、BioReference、PerkinElmer Inc.、LifeLabs、Abbott、BIO-HELIX、Cepheid、Eurofins Scientific など。 |
世界の遺伝性癌検査市場の定義
遺伝性癌とは、遺伝的変異が受け継がれることで起こる癌のことです。特定の遺伝子の有害な変異は、癌のリスク増加と関連しています。遺伝子検査により、癌を発症する生涯リスクを推定できます。これは、遺伝子、染色体、またはタンパク質の変異を調べることで行うことができます。遺伝子検査は、いくつかの種類の癌で利用できます。これには、乳癌、卵巣癌、結腸癌、甲状腺癌、前立腺癌、膵臓癌、皮膚癌、肉腫、腎臓癌、胃癌が含まれます。多くの医学的研究により、一般的な癌の 5% から 10% が遺伝性であると考えられています。遺伝子検査は、人が有害な遺伝子変異を持っているかどうかを判断するために行われます。これらの検査は、まだ癌にかかっていない家族が、癌感受性の代替物を持っていることがわかっている家族と同じ変異を受け継いでいるかどうかを判断するのにも役立ちます。
遺伝性癌検査の世界市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
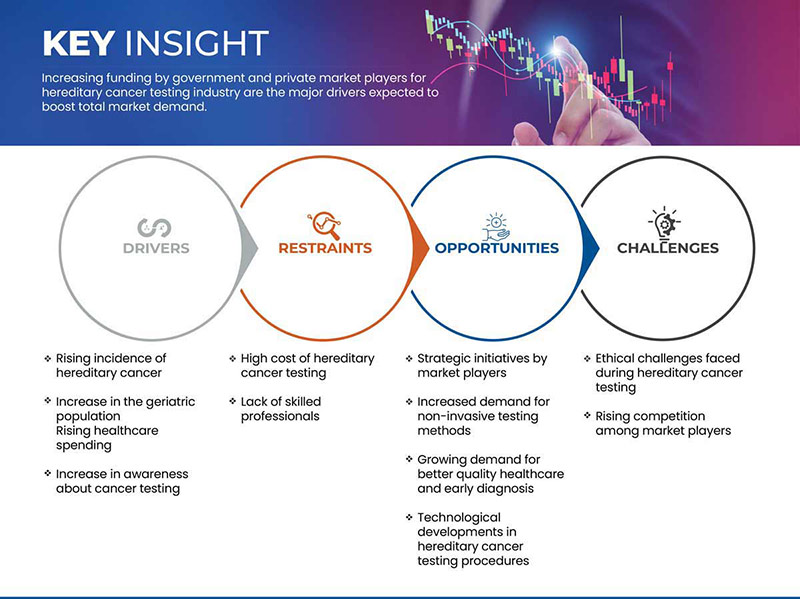
ドライバー
- 遺伝性癌の発生率上昇
がんは、細胞の制御されない増殖から生じます。がんは、細胞の成長と分裂を制御する遺伝情報(遺伝子)の有害な変化(突然変異)によって発生し、細胞が効果的に機能できなくなります。
遺伝性癌の場合、個人は片方の親から変異した成長制御遺伝子のコピーを受け継ぎ、もう一方の親から同じ遺伝子の有効なコピーを受け継ぎます。変異した遺伝子は「癌感受性遺伝子」とも呼ばれます。この癌感受性遺伝子は受け継がれるため、体内のすべての細胞に存在しますが、遺伝子の有効なコピーによって各細胞は正常に機能します。ただし、変異によって細胞内の遺伝子の機能コピーが損傷すると、細胞は成長を制御できなくなり、癌化します。したがって、癌遺伝子を受け継いだ個人は、生涯に特定の癌を発症する可能性が非常に高くなります。
したがって、遺伝性癌の発生率の上昇により、遺伝性癌検査の需要が増加し、世界的な遺伝性癌検査市場の成長の原動力となる可能性があります。
- 高齢者人口の増加
がんは高齢患者の病気になり得ます。世界中で、高齢者人口が増加しています。高齢者の遺伝性がんのリスクはさらに高くなります。高齢者人口の増加は、世界的な遺伝性がん検査市場の供給を改善する可能性があります。世界的な遺伝性がん検査市場の需要の急増が予想されます。 人口の高齢化は人口構造の再分配を引き起こし 、医療の将来に影響を与えます。間違いなく、がんのリスクは年齢とともに指数関数的に増加します。
世界最大の完全な家族構成と医学的に確認された癌データベースを使用した遺伝性癌の発生率と関連リスクを含む調査では、遺伝性癌に罹患した親または親戚がいない人の兄弟姉妹から生まれた 8 ~ 20 歳の人口では、遺伝性癌の発生率が約 2 倍高くなっていました。小腸癌、精巣癌、甲状腺癌、骨癌のリスクは 5 ~ 8 倍高くなっていました。
したがって、高齢者人口における癌発症率の増加は、世界的な遺伝性癌検査市場の成長の原動力になると予想されます。
拘束
- 遺伝性癌検査の高額な費用
遺伝性癌の検査には、非常に高度な技術を駆使した製品が用いられます。これらの製品の開発には、開発企業による厳格な研究開発が必要です。そのため、手順と製品のコストは依然として高く、それに比例して検査コストも増加します。検査キットは、膨大なリソースを必要とし、高額な医師、輸送、高価な医薬品を必要とするため、高価です。
- さらに、がん検査にも検査手順が使用されています。しかし、このような手順は非常に高価であり、合併症や長期的な結果の悪化につながる可能性があります。
したがって、高度なモダリティとテクノロジー製品を使用したがん検査のコストの高さは、世界的な遺伝性がん検査市場の成長に対する大きな抑制要因となる可能性があります。
機会
-
市場参加者による戦略的取り組み
The rise in the global hereditary cancer testing market increases the need for strategic business ideas. It includes a partnership, business expansion, and other development. The surge in demand for hereditary cancer treatment is significantly increasing the demand for diagnostic testing methods. The planned strategies allow the market players to align with the organization's functional activities to achieve set goals. It guides the company's discussions and decision-making in determining resource and budget requirements to accomplish objectives, thus increasing operational efficiency.
These strategic initiatives, such as product launches, agreements, and business expansion by the major market players, will boost the market growth and are expected to act as an opportunity for the global hereditary cancer testing market. The strategic initiatives are expected to aid in growth and improve the company's product portfolio, ultimately leading to more revenue generation. Hence, these strategic initiatives by the market players are expected to act as an opportunity for growth in the global hereditary cancer testing market.
Challenge
- Ethical Challenges Faced During Hereditary Cancer Testing
During hereditary cancer genetic testing, one of the significant ethical hindrances is health professionals' lack of basic knowledge about genetic testing and their lack of confidence in interpreting familial disease patterns. The challenge for healthcare providers is to provide sufficient information to support patient decision making-and evidence to support the reasoning behind any suggestions they might make.
The lack of reimbursement creates economic barriers to care. The process of hereditary cancer risk assessment and counseling is time-consuming, and it is unclear how best to document and bill for this service. Oncologists are often forced to navigate a potentially uncertain reimbursement environment for genetic testing, with various reimbursement policies among third-party payers.
Genetic testing for hereditary cancer elevates ethical matters, which can't be resolved with patients or their family members. The various aspects of ethical, cultural, and religious nature should not be a barrier to the act of hereditary cancer testing. All of these are issues to be solved. Therefore, ethical challenges during hereditary cancer testing are expected to challenge the market growth.
Post-COVID-19 Impact on Global hereditary cancer testing market
Many industries around the world have been disadvantaged over the past 18 months. This could be due to the major disruptions that their industrial and supply chain processes are experiencing due to various precautionary measures such as shutdowns and other restrictions that the facilities around the world have been following. The same places the global hereditary cancer testing market. Also, consumption demand has subsequently decreased as people now have more opportunities to exclude non-essential expenses from their budgets as most people's overall finances have been severely affected by the boom. These aforementioned factors can be expected to burden the revenue margin of the global hereditary cancer testing market during the forecast period.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities and product launch and strategic partnerships to improve the technology and test results involved in the transplant diagnostics market.
Recent Developments
- In July 2022, Helio Genomics and its business partner, Fulgent Genetics (FLGT) has announced that the American Medical Association (AMA) issued a new Category I Current Procedural Terminology (CPT) Proprietary Laboratory Analyses code for HelioLiver and broader adoption of advanced innovative surveillance tests for liver cancer in the U.S. This has helped the company to expand their product portfolio.
- In March 2022, Illumina, Inc. introduced the n vitro diagnostic (IVD) kit, a cancer RNA sequencer. The launch resulted in expansion of the sequencing product line, followed by post market approval.It is depicted to show a linear market growth.
Global Hereditary Cancer Testing Market Scope
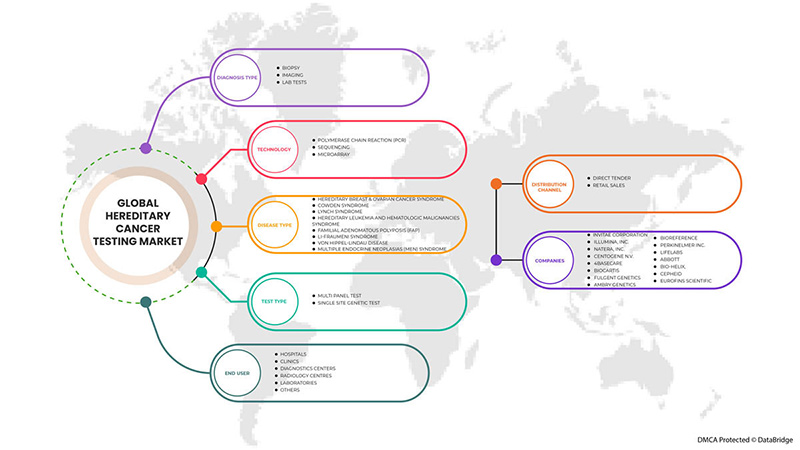
Global hereditary cancer testing market is segmented into test type, diagnosis type, technology, disease type, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
BY TEST TYPE
- MULTI PANEL TEST
- SINGLE SITE GENETIC TEST
On the basis of test type, the global hereditary cancer testing market is segmented into multi panel test and single site genetic test.
BY DIAGNOSIS TYPE
- BIOPSY
- IMAGING
- LAB TESTS
On the basis of diagnosis type, the global hereditary cancer testing market is segmented into biopsy, imaging, and lab tests.
BY TECHNOLOGY
- SEQUENCING
- POLYMERASE CHAIN REACTION (PCR)
- MICROARRAY
On the basis of technology, the global hereditary cancer testing market is segmented into sequencing, polymerase chain reaction (PCR), and microarray.
BY DISEASE TYPE
- HEREDITARY BREAST & OVARIAN CANCER SYNDROME
- COWDEN SYNDROME
- LYNCH SYNDROME
- HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROMES
- FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
- LI-FRAUMENI SYNDROME
- VON HIPPEL-LINDAU DISEASE
- MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROMES
On the basis of disease type, the global hereditary cancer testing market is segmented into hereditary breast & ovarian cancer syndrome, cowden syndrome, lynch syndrome, hereditary leukemia and hematologic malignancies syndromes, familial adenomatous polyposis (FAP), li-fraumeni syndrome, vol-hippel lindau disease, multiple endocrine neoplasias (MEN) syndrome.
BY END USER
- HOSPITALS
- CLINICS
- LABORATORIES
- RADIOLOGY CENTERS
- DIAGNOSTIC CENTERS
- OTHERS
On the basis of end user, the global hereditary cancer testing market is segmented into hospitals, clinics, laboratories, radiology centers, diagnostic centers, and others.
BY DISTRIBUTION CHANNEL
- DIRECT TENDER
- RETAIL SALES
On the basis of distribution channel, the global hereditary cancer testing market is segmented into direct tender, retail sales.
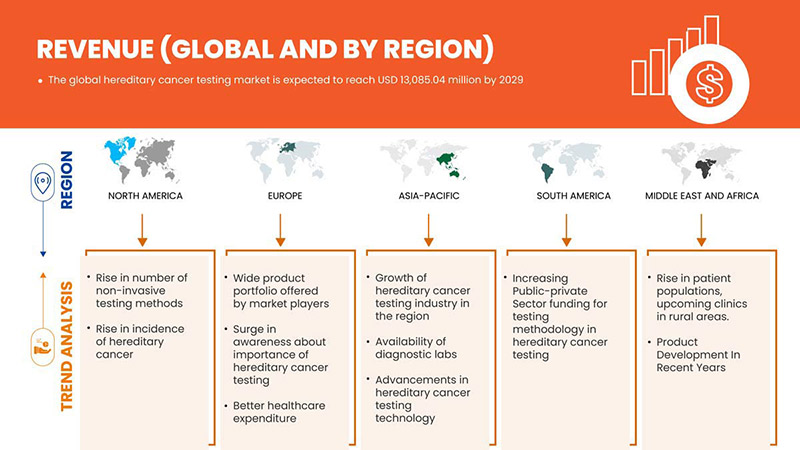
Global Hereditary Cancer Testing Market Regional Analysis/Insights
The global hereditary cancer testing market is analyzed and market size information is provided by country, test type, diagnosis type, technology, disease type, end user, and distribution channel.
The countries covered in this market report U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, and the rest of Europe, China, Japan, India, South Korea, Singapore, Thailand, Malaysia, Australia, Philippines, Indonesia, and the rest of Asia-Pacific, South Africa, Saudi Arabia, UAE, Egypt, Israel, and the rest of the Middle East and Africa, Brazil, Argentina, and the rest of South America.
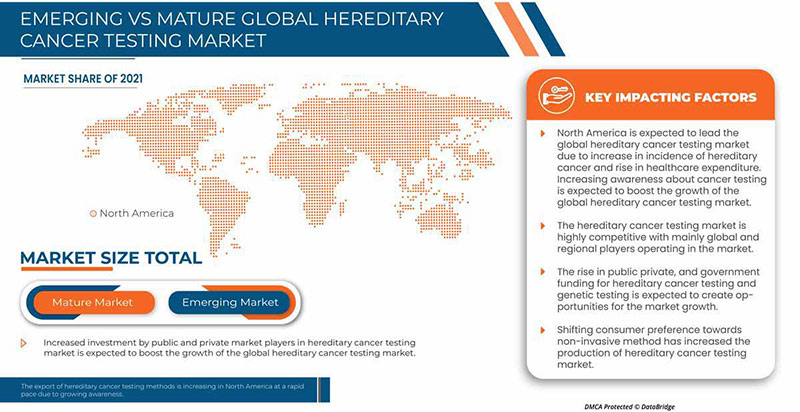
North America is dominating the market due to the increasing investment in R&D is expected to boost the market growth. The U.S. dominates North America region due to strong presence of key players Invitae Corporation, Illumina, Inc., Natera, Inc. and others. U.K. dominates Europe region due to the mass production of hereditary cancer tests and increasing demand from emerging markets and expansion of healthcare industries. China dominates Asia-Pacific region due to rise in cancer related diagnostic tests.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Hereditary Cancer Testing Market Share Analysis
世界の遺伝性癌検査市場の競争状況は、競合他社ごとに詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、技術ライフライン曲線などがあります。提供されている上記のデータ ポイントは、会社の世界の遺伝性癌検査市場への重点にのみ関連しています。
世界的な遺伝性癌検査市場で活動している主要企業としては、Invitae Corporation、Illumina, Inc.、Natera, Inc.、CENTOGENE NV、4baseCare、Biocartis、Fulgent Genetics、Ambry Genetics、BioReference、PerkinElmer Inc.、LifeLabs、Abbott、BIO-HELIX、Cepheid、Eurofins Scientific などが挙げられます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 GLOBAL HEREDITARY CANCER TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF HEREDITARY CANCER
6.1.2 INCREASE IN THE GERIATRIC POPULATION
6.1.3 RISING HEALTHCARE SPENDING
6.1.4 INCREASE IN AWARENESS ABOUT CANCER TESTING
6.2 RESTRAINTS
6.2.1 HIGH COST OF HEREDITARY CANCER TESTING
6.2.2 LACK OF SKILLED PROFESSIONALS
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.2 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
6.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE AND EARLY DIAGNOSIS
6.3.4 TECHNOLOGICAL DEVELOPMENTS IN HEREDITARY CANCER TESTING PROCEDURES
6.4 CHALLENGES
6.4.1 ETHICAL CHALLENGES FACED DURING HEREDITARY CANCER TESTING
6.4.2 RISING COMPETITION AMONG MARKET PLAYERS
7 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 MULTI PANEL TEST
7.3 SINGLE-SITE GENETIC TEST
8 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE
8.1 OVERVIEW
8.2 BIOPSY
8.2.1 NEEDLE BIOPSIES
8.2.2 ENDOSCOPIC BIOPSIES
8.2.3 LAPAROSCOPIC, THORACOSCOPIC, AND MEDIASTINOSCOPIC BIOPSY
8.2.4 LAPAROTOMY AND THORACOTOMY
8.2.5 OTHERS
8.3 IMAGING
8.3.1 MAGNETIC RESONANCE IMAGING (MRI)
8.3.2 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 NUCLEAR SCAN
8.3.5 ULTRASOUND
8.3.6 X-RAYS
8.4 LAB TESTS
8.4.1 BLOOD
8.4.2 URINE
8.4.3 OTHERS
9 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 POLYMERASE CHAIN REACTION (PCR)
9.3 SEQUENCING
9.4 MICRO ARRAY
10 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 HEREDITARY BREAST & OVARIAN CANCER SYNDROME
10.3 COWDEN SYNDROME
10.4 LYNCH SYNDROME
10.5 HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME
10.6 FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
10.7 LI-FRAUMENI SYNDROME
10.8 VON HIPPEL-LINDAU DISEASE
10.9 MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME
11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 DIAGNOSTIC CENTERS
11.5 RADIOLOGY CENTERS
11.6 LABORATORIES
11.7 OTHERS
12 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
13 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION
13.1 OVERVIEW
13.2 ASIA-PACIFIC
13.2.1 CHINA
13.2.2 JAPAN
13.2.3 SOUTH KOREA
13.2.4 INDIA
13.2.5 AUSTRALIA
13.2.6 SINGAPORE
13.2.7 THAILAND
13.2.8 MALAYSIA
13.2.9 INDONESIA
13.2.10 PHILIPPINES
13.2.11 REST OF ASIA-PACIFIC
13.3 NORTH AMERICA
13.3.1 U.S.
13.3.2 CANADA
13.3.3 MEXICO
13.4 EUROPE
13.4.1 GERMANY
13.4.2 FRANCE
13.4.3 U.K.
13.4.4 RUSSIA
13.4.5 ITALY
13.4.6 SPAIN
13.4.7 TURKEY
13.4.8 NETHERLANDS
13.4.9 SWITZERLAND
13.4.10 BELGIUM
13.4.11 REST OF EUROPE
13.5 SOUTH AMERICA
13.5.1 BRAZIL
13.5.2 ARGENTINA
13.5.3 REST OF SOUTH AMERICA
13.6 MIDDLE EAST AND AFRICA
13.6.1 SOUTH AFRICA
13.6.2 SAUDI ARABIA
13.6.3 U.A.E.
13.6.4 EGYPT
13.6.5 ISRAEL
13.6.6 REST OF THE MIDDLE EAST AND AFRICA
14 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ABBOTT
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 ILLUMINA, INC. (2021)
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PERKINELMER INC. (2021)
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 LIFELABS GENETICS
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 EUROFINS SCIENTIFIC (2021)
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 AMBRY GENETICS
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 BIOCARTIS
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 BIO-HELIX
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOREFERENCE (A SUBSIDIARY OF OPKO HEALTH, INC.) (2021)
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CENTOGENE N.V. (2021)
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 FULGENT GENETICS
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 INVITAE CORPORATION
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 NATERA, INC. (2021)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 4BASECARE.
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
表のリスト
TABLE 1 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 2 GLOBAL MULTI PANEL TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 GLOBAL SINGLE-SITE GENETIC TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 5 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 7 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 9 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 12 GLOBAL POLYMERASE CHAIN REACTION (PCR) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 GLOBAL SEQUENCING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 GLOBAL MICROARRAY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 16 GLOBAL HEREDITARY BREAST & OVARIAN CANCER SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 GLOBAL COWDEN SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 GLOBAL LYNCH SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 GLOBAL HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 GLOBAL FAMILIAL ADENOMATOUS POLYPOSIS (FAP) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 GLOBAL LI-FRAUMENI SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 GLOBAL VON HIPPEL-LINDAU DISEASE IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 GLOBAL MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 25 GLOBAL HOSPITALS CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 GLOBAL CLINICS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 GLOBAL DIAGNOSTIC CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 GLOBAL RADIOLOGY CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 GLOBAL LABORATORIES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 GLOBAL OTHERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 GLOBAL DIRECT TENDER IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 GLOBAL RETAIL SALES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 43 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 44 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 46 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 47 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 48 CHINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 50 CHINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 51 CHINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 52 CHINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 53 CHINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 54 CHINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 55 CHINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 57 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 58 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 59 JAPAN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 60 JAPAN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 61 JAPAN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 62 JAPAN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 63 JAPAN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 64 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 65 JAPAN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 66 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 67 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 69 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 70 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 71 SOUTH KOREA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 72 SOUTH KOREA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 73 SOUTH KOREA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 74 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 75 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 77 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 79 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 80 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 81 INDIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 82 INDIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 83 INDIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 84 INDIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 85 INDIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 86 INDIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 87 INDIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 88 INDIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 89 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 91 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 92 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 93 AUSTRALIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 94 AUSTRALIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 95 AUSTRALIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 96 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 97 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 98 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 99 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 100 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 101 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 102 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 103 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 104 SINGAPORE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 105 SINGAPORE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 106 SINGAPORE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 107 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 108 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 109 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 110 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 111 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 113 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 114 THAILAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 115 THAILAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 116 THAILAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 117 THAILAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 118 THAILAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 119 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 120 THAILAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 121 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 122 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 123 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 124 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 125 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 126 MALAYSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 127 MALAYSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 128 MALAYSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 129 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 130 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 131 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 132 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 133 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 135 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 136 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 137 INDONESIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 138 INDONESIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 139 INDONESIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 140 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 141 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 142 INDONESIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 143 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 144 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 145 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 146 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 147 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 148 PHILIPPINES BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 149 PHILIPPINES IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 150 PHILIPPINES LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 151 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 152 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 153 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 154 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 155 REST OF ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 157 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 158 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 159 NORTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 160 NORTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 161 NORTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 162 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 163 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 164 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 165 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 166 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 167 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 168 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 169 U.S. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 170 U.S. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 171 U.S. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 172 U.S. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 173 U.S. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 174 U.S. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 175 U.S. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 176 U.S. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 177 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 179 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 180 CANADA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 181 CANADA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 182 CANADA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 183 CANADA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 184 CANADA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 185 CANADA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 186 CANADA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 187 CANADA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 188 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 189 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 190 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 191 MEXICO HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 192 MEXICO BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 193 MEXICO IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 194 MEXICO LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 195 MEXICO HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 196 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 197 MEXICO HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 198 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 199 EUROPE HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 200 EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 201 EUROPE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 202 EUROPE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 203 EUROPE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 204 EUROPE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 205 EUROPE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 206 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 207 EUROPE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 208 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 209 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 210 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 211 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 212 GERMANY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 213 GERMANY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 214 GERMANY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 215 GERMANY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 216 GERMANY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 217 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 218 GERMANY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 219 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 220 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 221 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 222 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 223 FRANCE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 224 FRANCE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 225 FRANCE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 226 FRANCE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 227 FRANCE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 228 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 229 FRANCE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 230 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 231 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 232 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 233 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 234 U.K. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 235 U.K. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 236 U.K. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 237 U.K. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 238 U.K. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 239 U.K. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 240 U.K. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 241 U.K. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 243 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 244 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 245 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 246 RUSSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 247 RUSSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 248 RUSSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 249 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 250 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 251 RUSSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 252 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 253 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 254 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 255 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 256 ITALY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 257 ITALY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 258 ITALY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 259 ITALY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 260 ITALY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 261 ITALY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 262 ITALY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 263 ITALY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 264 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 265 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 266 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 267 SPAIN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 268 SPAIN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 269 SPAIN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 270 SPAIN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 271 SPAIN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 272 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 273 SPAIN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 274 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 275 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 276 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 277 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 278 TURKEY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 279 TURKEY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 280 TURKEY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 281 TURKEY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 282 TURKEY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 283 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 284 TURKEY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 285 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 286 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 287 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 288 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 289 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 290 NETHERLANDS BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 291 NETHERLANDS IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 292 NETHERLANDS LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 293 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 294 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 295 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 296 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 297 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 298 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 299 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 300 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 301 SWITZERLAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 302 SWITZERLAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 303 SWITZERLAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 304 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 305 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 306 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 307 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 310 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 311 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 312 BELGIUM BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 313 BELGIUM IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 314 BELGIUM LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 315 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 316 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 317 BELGIUM HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 318 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 319 REST OF EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 320 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 321 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 322 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 323 SOUTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 324 SOUTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 325 SOUTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 326 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 327 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 328 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 329 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 330 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 331 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 332 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 333 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 334 BRAZIL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 335 BRAZIL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 336 BRAZIL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 337 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 338 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 339 BRAZIL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 340 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 341 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 342 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 343 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 344 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 345 ARGENTINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 346 ARGENTINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 347 ARGENTINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 348 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 349 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 350 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 351 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 352 REST OF THE SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 353 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 354 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 355 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 356 MIDDLE EAST AND AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 357 MIDDLE EAST AND AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 358 MIDDLE EAST AND AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 359 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 360 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 361 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 362 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 363 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 364 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 365 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 366 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 367 SOUTH AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 368 SOUTH AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 369 SOUTH AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 370 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 371 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 372 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 373 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 374 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 375 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 376 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 377 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 378 SAUDI ARABIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 379 SAUDI ARABIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 380 SAUDI ARABIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 381 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 382 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 383 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 384 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 385 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 386 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 387 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 388 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 389 U.A.E. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 390 U.A.E. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 391 U.A.E. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 392 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 393 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 394 U.A.E. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 395 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 396 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 397 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 398 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 399 EGYPT HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 400 EGYPT BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 401 EGYPT IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 402 EGYPT LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 403 EGYPT HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 404 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 405 EGYPT HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 406 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 407 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 408 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 409 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 410 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 411 ISRAEL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 412 ISRAEL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 413 ISRAEL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 414 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 415 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 416 ISRAEL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 417 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 418 REST OF MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
図表一覧
FIGURE 1 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 2 GLOBAL HEREDITARY CANCER TESTING MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL HEREDITARY CANCER TESTING MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL HEREDITARY CANCER TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL HEREDITARY CANCER TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL HEREDITARY CANCER TESTING MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 GLOBAL HEREDITARY CANCER TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL HEREDITARY CANCER TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE GLOBAL HEREDITARY CANCER TESTING MARKET, AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 12 EXPANDING REPRODUCTIVE GENETIC HEALTH SPACE IS EXPECTED TO DRIVE THE GLOBAL HEREDITARY CANCER TESTING MARKET IN THE FORECAST PERIOD
FIGURE 13 MULTI PANEL TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL HEREDITARY CANCER TESTING MARKET IN 2022 & 2029
FIGURE 14 NORTH AMERICA IS THE FASTEST-GROWING MARKET FOR HEREDITARY CANCER TESTING MANUFACTURERS IN THE FORECAST PERIOD
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
FIGURE 16 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2021
FIGURE 17 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 18 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 19 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2021
FIGURE 21 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2022-2029 (USD MILLION)
FIGURE 22 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, CAGR (2022-2029)
FIGURE 23 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2021
FIGURE 25 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2020-2029 (USD MILLION)
FIGURE 26 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 27 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 28 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2021
FIGURE 29 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2022-2029 (USD MILLION)
FIGURE 30 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, CAGR (2022-2029)
FIGURE 31 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 32 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2021
FIGURE 33 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2020-2029 (USD MILLION)
FIGURE 34 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, CAGR (2022-2029)
FIGURE 35 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, LIFELINE CURVE
FIGURE 36 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2021
FIGURE 37 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 38 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 39 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 GLOBAL HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 41 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021)
FIGURE 42 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2022 & 2029)
FIGURE 43 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021 & 2029)
FIGURE 44 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 45 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 46 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 47 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 48 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 49 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 50 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 51 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 52 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 53 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 54 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 55 EUROPE HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 60 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 61 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 62 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 63 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 64 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 65 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 66 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 67 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 68 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 69 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 70 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 71 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 72 EUROPE HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 73 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。