世界の電子臨床アウトカム評価(eCOA)市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
世界の電子臨床アウトカム評価(eCOA)市場セグメンテーション、タイプ別(臨床医報告アウトカム評価(CLINRO)、患者報告アウトカム評価(PRO)、観察者報告アウトカム評価(OBSRO)、パフォーマンスアウトカム評価(PERFO))、モダリティ別(サイトベースソリューション、ウェブソリューション、ハンドヘルド)、エンドユーザー別(開発業務受託機関(CRO)、製薬・バイオテクノロジー企業、医療機器企業、病院・医療機関、コンサルティングサービス企業、学術研究機関、その他)、配信モード別(クラウドベースおよびウェブホスト型) - 2032年までの業界動向と予測
電子臨床アウトカム評価(eCOA)市場規模
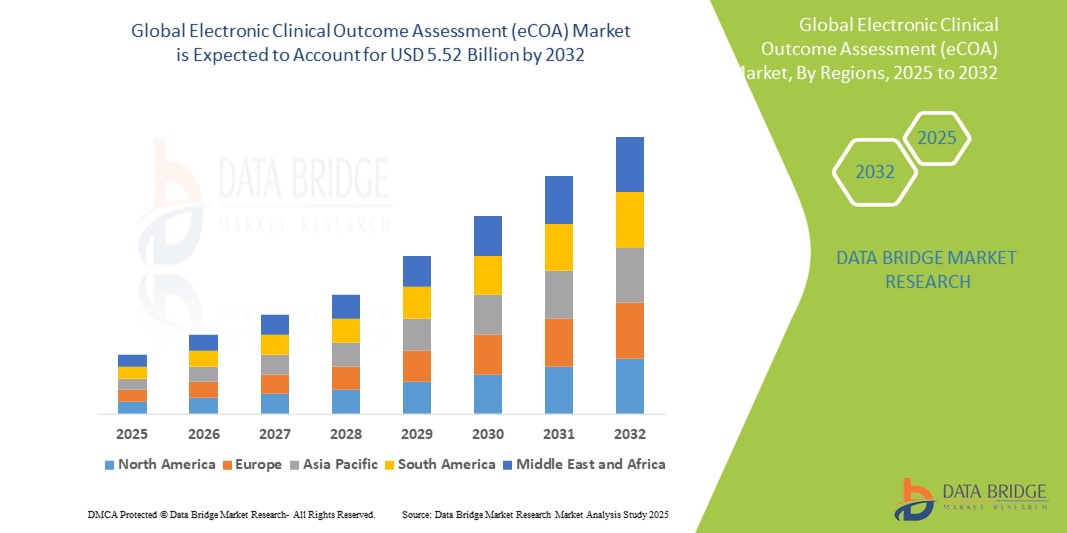
- 世界の電子臨床アウトカム評価(eCOA)市場規模は2024年に17億米ドルと評価され、予測期間中に15.80%のCAGRで成長し、2032年には55億2000万米ドル に達すると予想されています 。
- 市場の成長は主に、臨床試験や医療研究におけるデジタル技術の導入の増加によって推進され、より正確で効率的な患者データの収集とモニタリングを促進しています。
- さらに、リアルタイムの患者情報、規制遵守の向上、データ整合性の強化に対する需要の高まりにより、製薬会社、開発業務受託機関(CRO)、医療提供者全体でeCOAソリューションの導入が促進されています。
電子臨床アウトカム評価(eCOA)市場分析
- eCOAソリューションは、患者、介護者、または臨床医から直接臨床結果データを電子的に取得することを可能にし、そのデータ精度の向上、リアルタイムモニタリング機能、デジタルヘルスエコシステムとのシームレスな統合により、現代の臨床試験や医療研究においてますます重要な要素となっています。
- eCOAの需要の高まりは、主にデジタルヘルス技術の普及、患者中心の試験への重点の高まり、試験の効率とコンプライアンスを向上させるリモートでユーザーフレンドリーなデータ収集方法の好まれによって促進されています。
- 北米は、2024年に43.5%という最大の収益シェアで電子臨床アウトカム評価(eCOA)市場を支配しており、デジタル臨床試験ソリューションの早期導入、強力な製薬およびバイオテクノロジーセクター、電子データ収集をサポートする規制枠組みを特徴としています。米国では、モバイルおよびクラウドベースのプラットフォームに重点を置く既存のベンダーと新興技術プロバイダーの両方によるイノベーションによって大幅な成長が見込まれています。
- アジア太平洋地域は、臨床試験活動の増加、医療投資の増加、中国やインドなどの新興市場におけるデジタルツールの利点に対する認識の高まりにより、予測期間中に電子臨床アウトカム評価(eCOA)市場で最も急速に成長する地域になると予想されています。
- 患者報告アウトカム評価(PRO)セグメントは、治療効果と生活の質に関する患者の視点を捉えるという重要な役割を担っており、スポンサーや規制当局による優先順位が高まっていることから、2024年には48.5%の市場シェアを獲得し、電子臨床アウトカム評価(eCOA)市場を席巻するでしょう。
レポートの範囲と電子臨床アウトカム評価(eCOA)市場のセグメンテーション
|
属性 |
電子臨床アウトカム評価(eCOA)の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
電子臨床アウトカム評価(eCOA)市場動向
「AIと遠隔患者モニタリングによる臨床試験の効率向上」
- 世界のeCOA市場における重要かつ加速的なトレンドとして、臨床試験データ収集プラットフォームにおける人工知能(AI)と遠隔患者モニタリング技術の統合が進んでいます。こうした技術の融合により、臨床アウトカム評価の精度、適時性、そして患者中心性が大幅に向上しています。
- 例えば、MedidataやERTといった大手eCOAプロバイダーは、AIを活用した分析を導入し、患者報告データのパターンを特定することで、有害事象の早期発見と治験の意思決定の改善を可能にしています。同様に、ウェアラブルデバイスとeCOAプラットフォームを組み合わせることで、従来の治験実施施設への訪問に加えて、患者の健康指標を継続的にリアルタイムでモニタリングすることが可能になります。
- eCOAへのAI統合により、患者の服薬遵守に関する予測分析、データ品質の自動チェック、患者の異常反応に対するインテリジェントアラートなどの機能が可能になります。さらに、遠隔モニタリング機能により、患者は自宅から結果を報告するための便利で使いやすいインターフェースを利用でき、データの完全性とエンゲージメントが向上します。
- eCOAシステムと幅広いデジタルヘルスおよび臨床試験管理プラットフォームとのシームレスな統合により、スポンサーはデータ管理を一元化し、試験ワークフローを効率化できます。統合ダッシュボードを通じて、臨床チームは患者データ、治験実施施設のパフォーマンス、規制遵守をリアルタイムでモニタリングできます。
- よりインテリジェントで、接続性に優れ、患者に優しい臨床アウトカムソリューションへのこのトレンドは、臨床試験のデータ収集に対する期待を根本的に変化させています。その結果、Oracle HealthやCRF Healthなどの企業は、強化された予測機能とリモートデータキャプチャ機能を備えたAI対応のeCOAプラットフォームを開発しています。
- 製薬、バイオテクノロジー、医療機器の各分野では、治験の効率、データの正確性、患者の体験を重視する関係者が増えており、AIと遠隔患者モニタリングを統合したeCOAソリューションの需要が急速に高まっています。
電子臨床アウトカム評価(eCOA)市場の動向
ドライバ
「患者中心の試験とデジタルデータの正確性に対する需要の高まり」
- 患者中心の臨床試験への注目の高まりと、正確でリアルタイムのデジタルデータ収集の必要性の高まりが相まって、電子臨床結果評価(eCOA)ソリューションの需要の高まりの大きな原動力となっています。
- 例えば、2024年1月には、ダッソー・システムズ傘下のメディデータが、分散型試験における患者コンプライアンスとデータ品質の向上を目的として、eCOAプラットフォームにAIを活用した新たな機能強化を導入しました。主要業界プレーヤーによるこのようなイノベーションは、予測期間中のeCOA市場の成長を牽引すると予想されます。
- 製薬会社やバイオテクノロジー企業が臨床試験のプロセスを合理化し、市場投入までの時間を短縮することを目指している中、eCOAプラットフォームは、リアルタイムのデータキャプチャ、遠隔患者レポート、自動検証などの高度な機能を提供し、従来の紙ベースの方法に比べて大幅な改善をもたらします。
- さらに、分散型およびハイブリッド型の臨床試験モデルの採用が拡大するにつれ、eCOAは遠隔データ収集に不可欠な要素として位置づけられ、高い規制遵守基準を維持しながら患者のエンゲージメントを向上させています。
- eCOAプラットフォームは、電子データ収集、多言語サポート、ウェアラブルデバイスやモバイルアプリケーションとの統合を通じて試験の効率性を高めることができ、CRO、製薬会社、研究機関における導入を促進する重要な要素となっています。臨床試験の脱落率の低減とデータ整合性の向上への関心の高まりは、現代の臨床研究におけるeCOAソリューションの広範な導入をさらに後押ししています。
抑制/挑戦
「データプライバシー、規制遵守、そして高額な導入コストに関する懸念」
- データプライバシー、規制遵守、電子臨床アウトカム評価(eCOA)プラットフォームの初期導入コストの高さをめぐる懸念は、より広範な市場導入に大きな課題をもたらしている。
- eCOAシステムは、患者の健康に関する機密データを電子的に取得・送信するため、HIPAA、GDPR、21 CFR Part 11などの厳格なデータ保護規制の対象となり、スポンサーやCROにとってコンプライアンスが複雑でリソース集約的なものとなります。
- 例えば、複数の臨床試験スポンサーは、データのローカリゼーションルールに関する不確実性と、特に複数地域での試験における国境を越えたデータコンプライアンスの確保の複雑さのため、eCOAシステムへの完全な移行に慎重な姿勢を示している。
- これらの課題に対処するには、堅牢なデータセキュリティ基盤、定期的な監査、そしてグローバルなコンプライアンス基準の遵守が不可欠です。Oracle HealthやSignant Healthといった大手eCOAプロバイダーは、リスクを軽減し、治験関係者との信頼関係を維持するために、暗号化プラットフォームと規制関連トレーニングに多額の投資を行っています。
- さらに、eCOAシステムの導入には、ライセンス料、ハードウェア調達、スタッフトレーニング、システム統合など、初期費用が高額であることから、特に中小規模の研究機関にとって導入障壁となる可能性があります。データ精度の向上や試験期間の短縮といった長期的なメリットは広く認識されていますが、初期の経済的負担が、リソースが限られた機関における導入を阻む可能性があります。
- スケーラブルな価格モデル、クラウドベースの配信、そして安全でユーザーフレンドリーなプラットフォームの継続的なイノベーションを通じてこれらの課題を克服することは、臨床研究の分野全体でeCOAソリューションのより広範かつ持続的な採用を促進するために不可欠です。
電子臨床アウトカム評価(eCOA)市場の範囲
市場は、タイプ、モダリティ、エンドユーザー、配信モードに基づいてセグメント化されています。
- タイプ別
電子臨床アウトカム評価(eCOA)市場は、種類別に患者報告アウトカム(PRO)、医師報告アウトカム(ClinRO)、観察者報告アウトカム(ObsRO)、パフォーマンスアウトカム(PerfO)に分類されます。患者報告アウトカム(PRO)セグメントは、患者の経験、症状、治療結果に関する直接的な洞察を捉える患者中心のアプローチにより、2024年には48.5%と最大の市場収益シェアを獲得しました。PROツールは、患者が電子プラットフォームを通じてリアルタイムで健康データを直接報告できるようにすることで、データの精度と患者の関与を高め、最終的には臨床試験の質を向上させます。
臨床試験の複雑化と、正確かつ標準化されたデータ収集方法の必要性により、臨床医報告アウトカム(ClinRO)セグメントは予測期間中に大幅な成長が見込まれています。ClinROは、訓練を受けた医療専門家による評価を伴い、特に患者による自己報告が困難な場合に、臨床介入を評価するための客観的かつ信頼性の高いデータを提供します。
- モダリティ別
電子臨床アウトカム評価(eCOA)市場は、モダリティに基づいて、施設ベースのソリューション、Webソリューション、ハンドヘルドデバイスの3つに分類されます。Webソリューションセグメントは、ユーザーフレンドリーなインターフェース、容易なアクセス性、そして低い投資ニーズにより、2024年に最大の市場収益シェアを獲得しました。Webホスト型ソリューションは、クライアントデータをクラウドサーバーに保存し、基本的なコンピュータハードウェアとインターネット接続があればWeb経由でアクセスできるため、柔軟なカスタマイズが可能で、特定の顧客ニーズに合わせたカスタマイズが可能です。
ハンドヘルドデバイス分野は、臨床試験におけるモバイル技術の導入増加に牽引され、予測期間中に大幅な成長が見込まれています。ハンドヘルドデバイスはリアルタイムのデータ取得を容易にし、患者のコンプライアンスを向上させるため、分散型および遠隔型の臨床試験にとって魅力的な選択肢となります。
- エンドユーザー別
エンドユーザー別に見ると、電子臨床アウトカム評価(eCOA)市場は、製薬・バイオテクノロジー企業、開発業務受託機関(CRO)、医療機器企業、病院/医療機関、コンサルティングサービス企業、学術研究機関、その他に分類されます。製薬・バイオテクノロジー企業セグメントが市場を牽引し、2024年には50.66%の市場シェアを占めます。この優位性は、eCOAソリューションが医薬品開発プロセスにおけるデータ収集と分析の効率化、規制基準へのコンプライアンス確保、臨床試験データの精度向上において重要な役割を果たしていることに起因しています。
大手バイオ医薬品企業や医療機器企業による臨床研究管理のアウトソーシング増加の傾向に牽引され、CRO(契約研究機関)セグメントは予測期間中に大幅な成長が見込まれています。CROは、試験設計、被験者募集、データ収集、分析を含む包括的なサービスを提供し、eCOA(臨床開発・臨床開発)分野において不可欠な存在となっています。
配送方法別
電子臨床アウトカム評価(eCOA)市場は、提供形態に基づいて、クラウドベースとウェブホスト型のソリューションに分類されます。ウェブホスト型ソリューションは、クラウドベースソリューションに比べて費用対効果が高いことから、2025年には58.9%という最大の市場シェアを獲得しました。ウェブホスト型プラットフォームは、エンドユーザーにとって初期インフラ投資が少なく、製薬会社、CRO、医療機関の設備投資を削減します。
クラウドベースのソリューションセグメントは、その拡張性、柔軟性、そしてコスト効率の高さにより、予測期間中に大幅な成長が見込まれています。クラウドベースのプラットフォームは、臨床試験関係者が場所を問わず、データへのより容易かつ迅速なアクセスを可能にし、これは複数施設での試験において非常に重要です。
電子臨床アウトカム評価(eCOA)市場の地域分析
- 北米は、デジタル臨床試験ソリューションの早期導入、強力な製薬およびバイオテクノロジーセクター、電子データ収集をサポートする規制枠組みにより、2024年には電子臨床アウトカム評価(eCOA)市場で最大の収益シェア43.5%を獲得し、市場を支配しています。
- この地域は、臨床研究のデジタル変革をサポートする強力な規制枠組みの恩恵を受けており、製薬会社や契約研究機関がeCOAプラットフォームを導入してデータの精度と規制遵守を強化することを奨励しています。
- さらに、研究開発への積極的な投資、確立された医療インフラ、そして分散型かつ患者中心の臨床試験の早期導入が、市場の成長に大きく貢献しています。有力なeCOAソリューションプロバイダーとCROの存在は、電子臨床アウトカム評価ツールの地域展開をさらに加速させています。
米国電子臨床アウトカム評価(eCOA)市場インサイト
米国の電子臨床アウトカム評価(eCOA)市場は、臨床試験における米国のリーダーシップと臨床研究の急速なデジタル化に牽引され、2024年には北米で最大の収益シェアとなる79.6%を獲得しました。FDAなどの規制当局は、データ品質と患者エンゲージメントの向上を目的としたデジタルツールの活用を強く推奨しており、eCOAシステムの普及に貢献しています。さらに、分散型およびハイブリッド型の臨床試験モデルへのニーズの高まりにより、遠隔地でのリアルタイム患者データ収集プラットフォームの需要が高まっています。米国市場は、豊富な研究開発資金、製薬大手の存在感、そして高度な医療ITインフラの恩恵も受けています。
欧州の電子臨床アウトカム評価(eCOA)市場インサイト
欧州の電子臨床アウトカム評価(eCOA)市場は、臨床試験全体におけるリアルワールドエビデンス、患者中心主義、データ標準化に対する規制当局の重視の高まりを背景に、予測期間を通じて大幅なCAGRで拡大すると予測されています。EU全体で多言語対応かつ文化に適応したデジタルソリューションへの需要が高まっており、柔軟で拡張性の高いeCOAプラットフォームの導入が加速しています。さらに、学術研究における連携の増加と、デジタルヘルス変革を促進する政策が地域の成長を支えています。ドイツ、英国、フランスなどの国々は、それぞれの試験エコシステムにおける技術導入をリードしています。
英国における電子臨床アウトカム評価(eCOA)市場に関する洞察
英国の電子臨床アウトカム評価(eCOA)市場は、強力なバイオ医薬品研究開発部門とNHS(国民保健サービス)による積極的なデジタルヘルス戦略に支えられ、予測期間中に注目すべきCAGRで成長すると予想されています。分散型試験の増加と電子データ収集に関する規制の明確化は、市場でのeCOAの普及を促進しています。高度な臨床研究環境と医療情報科学への多額の投資により、英国ではコンプライアンスの確保、患者エンゲージメントの向上、そして効率的なアウトカム追跡を可能にするeCOA技術の急速な普及が進んでいます。
ドイツにおける電子臨床アウトカム評価(eCOA)市場インサイト
ドイツの電子臨床アウトカム評価(eCOA)市場は、臨床試験における卓越性と厳格なデータ保護法に対する評価を背景に、予測期間中に大幅なCAGRで拡大すると予想されています。ドイツの規制当局は臨床データの信頼性とセキュリティを重視しており、治験依頼者やCROは安全で検証済みのeCOAソリューションへの投資を促しています。さらに、ドイツでは第I相~IV相試験におけるリアルタイムデータキャプチャの需要が高まっており、強力なヘルスケアITインフラも整備されているため、医療機器研究および医薬品研究におけるeCOA技術のさらなる統合が促進されています。
アジア太平洋地域の電子臨床アウトカム評価(eCOA)市場インサイト
アジア太平洋地域の電子臨床アウトカム評価(eCOA)市場は、中国、インド、韓国、日本などの国々における臨床研究活動の活発化と医療のデジタル変革に牽引され、2025年から2032年の予測期間中に最も高いCAGRで成長すると見込まれています。多国籍試験の拡大と多様な患者層への対応が、この地域の成長を支えています。デジタルヘルスプラットフォーム導入に対する政府の優遇措置と、モバイルベースのソリューションに対する需要の高まりにより、eCOAの導入は都市部および準都市部においてより現実的かつ広範囲に広がっています。CROとグローバル製薬企業との地域パートナーシップは、eCOAの導入をさらに推進しています。
日本における電子臨床アウトカム評価(eCOA)市場インサイト
日本の電子臨床アウトカム評価(eCOA)市場は、技術の高度化、人口の高齢化、そして臨床試験における質の高いデータの重要性を背景に、急速に成長しています。日本の規制当局であるPMDAは、デジタルエンドポイントとリモートデータキャプチャツールへの積極的な姿勢を強めています。また、在宅および外来での臨床試験の増加も市場形成に影響を与えており、正確で患者に優しいeCOAシステムのニーズが高まっています。より広範なeClinicalエコシステムやAIを活用した患者エンゲージメントツールとの統合により、さらなる成長が期待されます。
インドにおける電子臨床アウトカム評価(eCOA)市場に関する洞察
インドの電子臨床アウトカム評価(eCOA)市場は、臨床試験活動の急増、テクノロジーに精通した人口、そして医薬品製造能力の拡大に後押しされ、2024年にはアジア太平洋地域最大の市場収益シェアを占めました。インドの費用対効果の高いCRO環境と、医療デジタル化を支援する政府の政策は、世界的なスポンサー企業による国内臨床試験へのeCOAツール導入を促進しています。スマートフォンの普及率向上、遠隔医療の拡大、そして都市部および準都市部におけるインターネット接続の改善により、モバイルベースおよびクラウドベースのeCOAプラットフォームはよりアクセスしやすく、広く採用されています。
電子臨床アウトカム評価(eCOA)の市場シェア
電子臨床結果評価 (eCOA) 業界は、主に次のような定評のある企業によって主導されています。
- IQVIA(米国)
- クラリオ(米国)
- メディデータ(米国)
- ヴィーヴァシステムズ(米国)
- アース・リソーシズ・テクノロジー (米国)
- オラクル・ヘルス・サイエンス(米国)
- YPrime, LLC(米国)
- アリスグローバルLLC(米国)
- カストルEDC(オランダ)
- eClinicalWorks(米国)
- メドリオ社(米国)
- クリンワン(米国)
- シグナント・ヘルス(米国)
- Clinical Ink, Inc.(米国)
- Curebase, Inc.(米国)
- カイエンティス(フランス)
- Calyx(英国)
- データキューブド・ヘルス(米国)
- HealthDiary, Inc.(米国)
世界の電子臨床アウトカム評価(eCOA)市場の最新動向
- 2025年5月、Clario(米国)はWCG Clinical(米国)のeCOA事業を買収しました。これは、特に神経科学臨床試験におけるデジタルエンドポイントデータソリューションにおけるリーダーシップを強化するための戦略的な動きです。この買収により、Clarioの包括的なエンドポイントデータプラットフォームが拡張され、複雑な試験環境へのサポートが強化され、急速に進化するeCOA市場における地位をさらに強固なものにします。
- 2025年5月、Critical Path Institute(米国)は、「eCOA:共により良い未来を築く」イニシアチブを継続し、スポンサー、テクノロジーベンダー、規制当局の連携を目指しました。この共同取り組みは2025年3月まで継続され、競争前のベストプラクティスとeCOAデータ収集のための共通用語の確立、標準化の促進、そして多様な地域における導入の加速に重点を置いています。
- 2023年11月、Clinical InkはObserviaのSPUR行動診断ツールを導入し、患者エンゲージメントスイートを強化しました。この統合により、行動評価とライフスタイル修正、eCOA、eSource、デジタルバイオマーカーが統合され、患者の行動をより包括的に理解し、試験結果を向上させることを目指しています。
- 2023年10月、Clarioは分散型臨床試験(DCT)サービスプロバイダーであるTrial Dataと戦略的提携を締結しました。この提携により、Clarioは中国の臨床試験分野におけるプレゼンスを強化し、両社の専門知識を結集することで最先端の分散型試験ソリューションを提供し、地域における患者中心のアプローチを推進します。
- 2022年12月、eCOA臨床試験テクノロジー企業であるSuvoda LLCは、電子臨床アウトカム評価(eCOA)設計ツールキットを発表しました。このツールキットは、Suvoda IRTおよびeConsentとスムーズに統合できるように設計されており、eCOA導入におけるこれまでの問題点を解消し、設計プロセスの合理化を目指しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。