欧州キャリアスクリーニング市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
16.92 Billion
USD
48.60 Billion
2025
2033
USD
16.92 Billion
USD
48.60 Billion
2025
2033
| 2026 –2033 | |
| USD 16.92 Billion | |
| USD 48.60 Billion | |
|
|
|
|
欧州キャリアスクリーニング市場セグメンテーション:検査タイプ別(分子スクリーニング検査および生化学スクリーニング検査)、疾患タイプ別(嚢胞性線維症、テイ・サックス病、ゴーシェ病、鎌状赤血球症、脊髄性筋萎縮症、その他の常染色体劣性遺伝性疾患)、病状別(呼吸器疾患、血液疾患、神経疾患、その他)、技術別(DNAシーケンシング、ポリメラーゼ連鎖反応、マイクロアレイ、その他)、最終用途別(病院、基準検査機関、医師の診療所およびクリニック、その他) - 2033年までの業界動向および予測
欧州キャリアスクリーニング市場規模
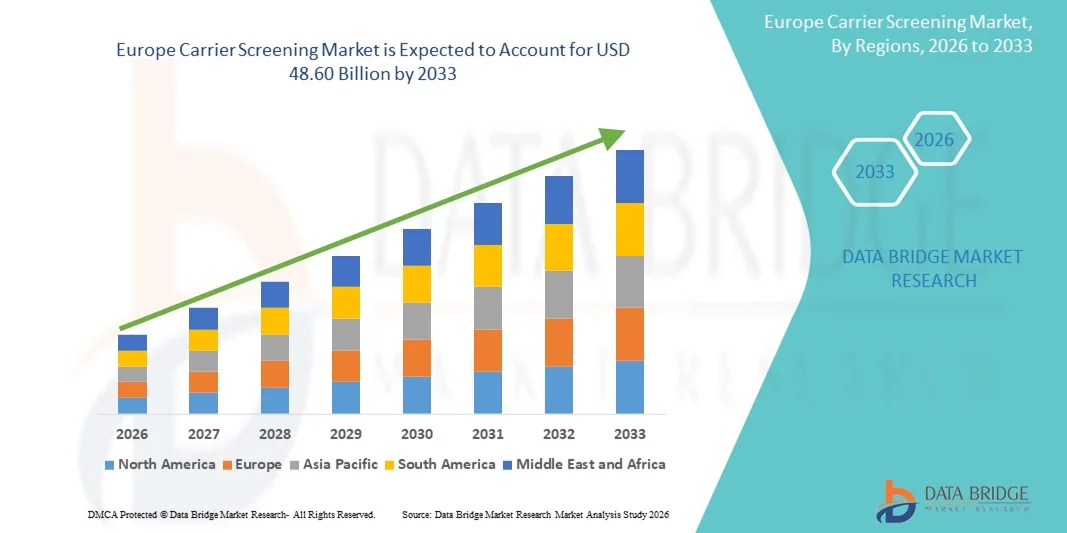
- ヨーロッパのキャリアスクリーニング市場規模は2025年に169.2億米ドルと評価され、予測期間中に14.00%のCAGRで成長し、2033年までに486億米ドルに達すると予想されています。
- 市場の成長は、遺伝性疾患に対する意識の高まりと、将来の親における保因者状態の早期発見への需要の高まりに大きく牽引されています。分子生物学的および生化学的スクリーニング技術の進歩と、より広範な保因者パネルの利用可能性により、より正確で包括的かつ迅速な検査が可能になり、病院、診療所、診断検査室における導入が促進されています。
- さらに、パーソナライズされたヘルスケアと生殖計画に対する消費者の嗜好の高まりと、自宅や検査室でのキャリアスクリーニングソリューションの利便性により、キャリアスクリーニングは出生前および妊娠前ケアにおける標準的な方法となりつつあります。これらの要因が重なり、キャリアスクリーニングサービスの普及が加速し、市場の拡大を大きく後押ししています。
欧州キャリアスクリーニング市場分析
- キャリアスクリーニングは、常染色体劣性疾患およびX連鎖疾患の遺伝子変異を持つ個人を特定するもので、家族計画の決定を情報に基づいて導き、遺伝性疾患の伝染リスクを減らすことができるため、臨床および家庭環境の両方で予防的生殖医療の重要な要素になりつつあります。
- キャリアスクリーニングの需要の高まりは、主に次世代シーケンシングなどの技術の進歩、拡大した多民族パネルの採用の増加、遺伝子検査を推進する政府や医療の取り組みの増加、早期発見と予防ケアの重要性に関する消費者と医療提供者の意識の高まりによって促進されています。
- ドイツは、強力な医療インフラ、高度な診断能力、出生前および妊娠前ケアにおける遺伝子検査の採用率の高さにより、キャリアスクリーニング市場を支配しました。
- 英国は、遺伝性疾患に対する意識の高まり、出生前検査および妊娠前検査プログラムの拡大、次世代スクリーニング技術の採用により、予測期間中にキャリアスクリーニング市場で最も急速に成長する国になると予想されています。
- 分子スクリーニング検査分野は、遺伝子変異の検出精度の高さと、遺伝性疾患の早期発見を求める将来の親の間での導入拡大により、62.8%の市場シェアを占め、市場を席巻しました。分子検査は、1回の検査で複数の疾患の保因者を特定し、包括的な結果を提供し、繰り返し検査の必要性を減らすことができるため、高く評価されています。医療提供者は、その信頼性と多様な集団における強力な臨床的妥当性から、分子スクリーニングを強く推奨しています。次世代シーケンシングパネルや標的変異解析の利用可能性の高まりは、臨床現場における分子検査の優位性をさらに強化しています。
レポートの範囲とキャリアスクリーニング市場のセグメンテーション
|
特性 |
キャリアスクリーニングの主要市場インサイト |
|
対象セグメント |
|
|
対象国 |
ヨーロッパ
|
|
主要市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
Data Bridge Market Research がまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、患者の疫学、パイプライン分析、価格分析、規制の枠組みも含まれています。 |
欧州キャリアスクリーニング市場の動向
拡大型および在宅型キャリアスクリーニングパネルの導入増加
- 遺伝性疾患への意識の高まりと積極的な生殖医療の必要性を背景に、キャリアスクリーニング市場では、拡張型および在宅型のスクリーニングパネルへの需要が高まっています。これらのパネルは、複数の遺伝性疾患を検出できる包括的な検査を提供し、十分な情報に基づいた家族計画の決定を可能にします。
- 例えば、ミテラ社の「Peaches & Me」と「23 Pears」の在宅検査キットは、自宅で複数の疾患のキャリアスクリーニングを実施できるため、消費者のアクセス性を高め、早期導入を促進します。こうした在宅ソリューションは、医療機関への受診の必要性を軽減し、テクノロジーに精通し健康志向の高い人々にとって便利な選択肢となります。
- 高度な分子・生化学技術は検査精度を向上させ、ターンアラウンドタイムを短縮することで、より信頼性の高い結果をもたらします。これらの技術革新は、個別化されたリスク評価をサポートし、医療提供者が検査結果に基づいた的確なカウンセリングを提供することを可能にします。
- 人口特異的かつ多民族的な保因者スクリーニングパネルの利用可能性が高まっており、多様な人口統計グループへの導入が促進されています。さらに、これらのパネルは希少な遺伝性疾患のより広範な検出を可能にするため、包括的な生殖保健管理においてますます重要になっています。
- デジタルレポートと遠隔遺伝カウンセリングをキャリアスクリーニングサービスと統合することで、ユーザーエクスペリエンスが向上します。こうしたイノベーションにより、患者は検査結果をオンラインで安全に受け取り、専門家の指導を受けることができるため、検査に対する消費者の信頼が高まります。
- 技術の進歩、利便性、そしてより広範なアクセスの組み合わせが市場を形成し、拡張パネルと在宅パネルが予防的生殖医療の中心的存在として位置づけられています。この傾向は、臨床、消費者、そして遠隔医療の分野全体にわたって成長を持続させると予想されます。
欧州キャリアスクリーニング市場の動向
推進要因
遺伝性疾患への意識の高まり
- 将来の親、医療従事者、そして政策立案者の間で遺伝性疾患に対する意識が高まっていることは、キャリアスクリーニング市場の重要な推進力となっています。遺伝性リスクへの理解が深まることで、早期検査と十分な情報に基づいた生殖に関する意思決定が促進されます。
- 例えば、NateraやFulgent Geneticsといった企業は、キャリアパネルの拡充や啓発キャンペーンを実施し、消費者に早期発見のメリットを啓発しています。さらに、これらの取り組みは、病院、診療所、在宅検査サービスにおける導入を促進し、市場浸透の拡大にもつながっています。
- 予防医療と個別化医療への関心が高まるにつれ、医療制度はキャリアスクリーニングを妊娠前および出生前ケアの日常プログラムに統合するようになっています。こうした統合により、リスク要因の早期発見が促進され、子孫における遺伝性疾患の発生リスクが低減します。
- 教育活動、ソーシャルメディアによる啓発活動、遺伝カウンセリングサービスにより、キャリアスクリーニングに対する理解と受容がさらに深まります。さらに、これらの取り組みにより、将来の親が十分な情報に基づいた生殖に関する選択を行えるようになり、市場の成長を加速させています。
- 生殖医療に関する政府プログラムや民間団体からの支援と、スクリーニング技術の進歩が相まって、スクリーニングの普及は引き続き促進されています。こうした協調的な取り組みにより、より広範なアクセスが確保され、キャリアスクリーニングが生殖医療の標準的な要素として確立されます。
制約/課題
高コストと限られた保険適用
- 包括的なキャリアスクリーニング検査の高コストは、市場導入の大きな障壁となっています。このようなコストは、価格に敏感な人々のアクセスを制限し、臨床分野と消費者向け分野の両方で全体的な普及を減少させる可能性があります
- 例えば、InvitaeやMyriad Geneticsといった企業が提供する拡張パネルは依然として高価であり、アクセスが制限され、財政資源が限られている地域では導入が進まない状況となっています。さらに、より広範な疾患をカバーするプレミアム検査ソリューションの普及も制限されています。
- 医療費の自己負担が大部分を占める国では、償還と保険適用の制限が経済的な問題を悪化させています。こうした制約により、多くの将来の親にとって高度な検査サービスを受けることが困難になっています。
- 基本的な検査オプションはより手頃な価格ですが、より高い精度とより広範な病状のカバー範囲を提供するプレミアムパネルは、依然としてユーザーが検査を選択することを躊躇させる可能性があります。さらに、保険会社間で一貫性のない適用範囲のポリシーは、キャリアスクリーニングサービスへの均一なアクセスを妨げています。
- これらの障壁を克服するには、検査費用の削減、保険適用範囲の拡大、そしてキャリアスクリーニングの価値に関する消費者への啓蒙活動といった取り組みが必要です。こうした財政面および保険適用範囲の制約に対処することは、先進国市場と新興国市場の両方において、公平なアクセスと持続的な成長を確保するために不可欠です。
欧州キャリアスクリーニング市場の展望
市場は、検査の種類、病気の種類、病状、技術、最終用途に基づいて分類されています。
- テストの種類別
検査の種類に基づいて、キャリアスクリーニング市場は、分子スクリーニング検査と生化学スクリーニング検査に分類されます。分子スクリーニング検査セグメントは、遺伝子変異の検出精度の高さと、遺伝性疾患の早期発見を求める将来の親の間での導入増加に牽引され、2025年には62.8%という最大の市場収益シェアで市場を席巻しました。分子検査は、1回の検査で複数の疾患のキャリアを特定し、包括的な結果を提供し、繰り返し検査を行う必要性を減らすことができるため、好まれています。医療提供者は、その信頼性と多様な集団における強力な臨床検証のために、分子スクリーニングを強く推奨しています。次世代シーケンシングパネルと標的変異解析の利用可能性が高まるにつれ、臨床現場での分子検査の優位性がさらに強化されています。
生化学スクリーニング検査分野は、費用対効果が高く迅速なスクリーニング方法の継続的な研究に支えられ、2026年から2033年にかけて19.4%という最も高い成長率を記録すると予想されています。例えば、NateraやInvitaeといった企業は、より広範な疾患を網羅するよう生化学スクリーニングパネルを拡充し、より簡略化されたサンプル採取プロセスとより迅速な処理時間を提供しています。インフラ要件が低いため、高度な分子生物学的検査へのアクセスが限られている地域では、生化学検査の導入がますます進んでいます。早期発見と予防医療に対する臨床医と患者の意識の高まりも、生化学検査の導入を後押ししています。
- 疾患別
疾患別に見ると、キャリアスクリーニング市場は嚢胞性線維症、テイ・サックス病、ゴーシェ病、鎌状赤血球症、脊髄性筋萎縮症、その他の常染色体劣性遺伝疾患に分類されます。嚢胞性線維症セグメントは、いくつかの集団における嚢胞性線維症キャリアの有病率の高さと、定期的なスクリーニングを推奨するガイドラインの確立により、2025年には28.5%という最大の市場収益シェアで市場を支配しました。嚢胞性線維症スクリーニングは、家族計画に重要な情報を提供し、重篤な遺伝性疾患の遺伝リスクを軽減します。検査室および臨床ネットワークは、嚢胞性線維症の変異を含むパネルに多額の投資を行っており、市場リーダーシップを強化しています
脊髄性筋萎縮症(SMA)分野は、SMAの発症率増加と早期診断技術の飛躍的進歩に牽引され、2026年から2033年にかけて18.7%という最も高いCAGR(年平均成長率)を達成すると予想されています。例えば、Fulgent GeneticsやBlueprint Geneticsといった企業は、SMA検出を含むキャリアパネルの拡充を図り、早期介入戦略を推進しています。SMA治療法や新生児スクリーニングプログラムへの認知度の高まりも、SMAの導入を後押ししています。医療提供者は、SMA検査が早期治療の成果と生活の質に与える影響の大きさから、SMA検査をますます重視するようになっています。
- 病状別
病状に基づいて、キャリアスクリーニング市場は、肺疾患、血液疾患、神経疾患、その他に分類されます。肺疾患セグメントは、嚢胞性線維症やその他の遺伝性肺疾患などの疾患の有病率の高さに牽引され、2025年には31.2%という最大の市場収益シェアを占め、市場を席巻しました。肺疾患スクリーニングは、早期診断、キャリア特定、そして個別カウンセリングに不可欠です。検査機関は、複数の肺疾患を同時に対象とする包括的なパネルを開発しており、出生前および妊娠前ケアの現場での検査の採用が増加しています。
神経疾患セグメントは、神経遺伝性疾患への意識の高まりと、SMA、テイ・サックス病、および関連疾患を検出できる診断技術の進歩に支えられ、2026年から2033年にかけて20.1%という最も高い成長率を記録すると予想されています。例えば、Invitaeの神経疾患スクリーニングパネルは、キャリアを早期に発見できるため、クリニックや病院での導入が進んでいます。神経遺伝学研究の進歩と遺伝子検査の保険適用拡大は、需要をさらに押し上げると予想されます。臨床医は、高リスク集団に対して神経疾患検査を推奨する傾向が高まっており、市場の成長を加速させています。
- 技術別
技術に基づいて、キャリアスクリーニング市場はDNAシーケンシング、ポリメラーゼ連鎖反応(PCR)、マイクロアレイ、その他に分類されます。DNAシーケンシング分野は、高い精度、拡張性、そして複数の遺伝子にわたる幅広い変異を同時に検出する能力により、2025年には55.6%という最大の市場収益シェアで市場を支配しました。DNAシーケンシングは、出生前および妊娠前のキャリアスクリーニングの両方で広く好まれており、包括的な洞察とカウンセリング結果の改善を可能にします。次世代シーケンシング(NGS)の採用により、DNAベースのキャリア検査の効率と費用対効果がさらに向上しました
PCR分野は、迅速な検出能力、コスト効率、そして標的変異解析への適用性の高さを背景に、2026年から2033年にかけて19.2%という最も高いCAGR(年平均成長率)を達成すると予想されています。例えば、GeneDxやMyriad Geneticsといった企業は、高頻度変異を迅速に検出するために、PCRベースのスクリーニングサービスを拡大しています。PCR技術は、小規模な研究室やNGSインフラが限られている地域での採用が引き続き増加しています。PCRアッセイの導入の容易さと高感度は、日常的なキャリア検出プログラムに適しており、さらなる成長を牽引しています。
- 最終用途別
キャリアスクリーニング市場は、最終用途に基づいて、病院、リファレンスラボ、診療所・クリニック、その他に分類されます。病院セグメントは、出生前ケア、妊娠前カウンセリング、遺伝子検査サービスへのキャリアスクリーニングの統合により、2025年には42.3%という最大の市場収益シェアを獲得し、市場を席巻しました。病院は、院内ラボ機能と患者への統合ケアパスウェイの提供能力から恩恵を受けており、これが採用率を高めています。大規模な病院ネットワークや大学病院は、診断企業と提携してキャリアスクリーニングパネルを拡充し、包括的な患者ケアを確保することが増えています。
リファレンスラボラトリー分野は、ハイスループット遺伝子検査サービスの拡大に牽引され、2026年から2033年にかけて21.5%という最も高い成長率を記録すると予想されています。例えば、Quest DiagnosticsやLabcorpといったラボラトリーは、複数の病院や診療所に効率的にサービスを提供するために、高度なキャリアスクリーニングプラットフォームへの投資を行っています。また、リファレンスラボラトリーは、自動化やマルチプレックス検査を活用して、処理時間と精度を向上させています。費用対効果の高い大規模な検査ソリューションを提供できることから、リファレンスラボラトリーはキャリアスクリーニング市場において急成長を遂げている分野の一つとなっています。
欧州キャリアスクリーニング市場の地域分析
- ドイツは、強力な医療インフラ、高度な診断能力、出生前および妊娠前ケアにおける遺伝子検査の普及率の高さにより、2025年に最大の収益シェアを獲得し、キャリアスクリーニング市場を支配しました。
- 予防医療への国の重点的な取り組みと遺伝性疾患に対する広範な認識が相まって、キャリアスクリーニングサービスの利用が加速しています。大手診断企業の強力な存在、分子・生化学スクリーニング技術の継続的な研究開発、そして病院や研究機関との連携が、市場拡大をさらに加速させています。
- ドイツは、キャリアスクリーニングを標準的な臨床プロトコルに統合し、患者カウンセリングを改善し、個別化された医療を支援することに重点を置いており、地域市場における主導的地位を強化している。
英国のキャリアスクリーニング市場の洞察
英国市場は、遺伝性疾患への意識の高まり、出生前・妊娠前検査プログラムの拡大、次世代スクリーニング技術の導入を背景に、2026年から2033年にかけて欧州のキャリアスクリーニング市場において最も高いCAGRを記録すると予測されています。早期発見を促進する政府および医療機関の取り組みの強化、そして国内検査機関と国際的な診断企業との戦略的提携が、市場の需要を牽引しています。例えば、NateraやInvitaeといった企業は、NHS傘下の病院と提携し、キャリアスクリーニングへのアクセス拡大に取り組んでいます。英国は、患者の転帰改善、高度な遺伝子検査へのアクセス向上、そして予防医療の支援に重点を置いており、この地域で最も急速に成長する市場となっています。
フランスのキャリアスクリーニング市場の洞察
フランスは、医療サービス、不妊治療クリニック、出生前ケアプログラムの拡大、そして包括的なキャリアスクリーニングパネルの導入増加に支えられ、2026年から2033年にかけて着実な成長を遂げると見込まれています。遺伝性疾患の早期診断、予防医療、分子技術の統合に重点を置くフランスは、正確性、幅広い疾患のカバー範囲、そして信頼性の高いカウンセリング結果を提供するキャリア検査サービスの需要を促進しています。高度な診断ラボへの投資増加と、生殖保健プログラムに対する政府の強力な支援が相まって、国内のクリニックや病院における導入率が向上しています。フランスの診断プロバイダーと国際企業との連携により、技術統合とサービスの質がさらに向上しています。予防医療と業務効率の向上に向けたフランスの取り組みは、欧州地域における安定した市場見通しを支えています。
欧州キャリアスクリーニング市場シェア
キャリアスクリーニング業界は、主に、次のような定評のある企業によって主導されています。
- ユーロフィン・サイエンティフィック(ルクセンブルク)
- インビテ・コーポレーション(米国)
- オプコ・ヘルス社(米国)
- ルミネックス・コーポレーション(米国)
- フルジェント・ジェネティクス(米国)
- クエスト・ダイアグノスティクス(米国)
- セマフォー・オプコ(米国)
- ミリアド・ジェネティクス(米国)
- イルミナ(米国)
- サーモフィッシャーサイエンティフィック(米国)
- メドゲノム(米国)
- ミリアド・ジェネティクス(米国)
- ナテラ(米国)
- ジーン・バイ・ジーン(米国)
- ラボラトリー・コーポレーション・オブ・アメリカ・ホールディングス(米国)
- マウントサイナイ・ゲノミクス社(米国)
- オトジェネティクス・コーポレーション(米国)
欧州キャリアスクリーニング市場の最新動向
- 2024年10月、ミリアド・ジェネティクスは500以上の遺伝子を網羅する「Foresight Plus」キャリアスクリーニングパネルの発売を発表しました。この拡張パネルにより、希少かつ頻度の低い遺伝性疾患をより包括的に検出できるようになり、臨床医や将来の親となる人々が潜在的な遺伝性リスクについてより深い洞察を得ることができます。この発売は、より広範で網羅性の高いスクリーニングソリューションへのトレンドを後押しし、キャリア検査の臨床的有用性を高め、病院、不妊治療クリニック、専門診断センターにおける導入を促進するものです。
- 2024年1月、ナテラはキャリアスクリーニングと非侵襲性出生前検査サービスを含むインビテの生殖医療ポートフォリオを5,250万ドルで買収しました。この戦略的買収により、ナテラは生殖遺伝学市場における地位を強化し、統合されたエンドツーエンドのスクリーニングソリューションを提供できるようになります。この買収により、臨床ワークフローの合理化、高度なキャリア検査へのアクセス向上、そして包括的な生殖リスク評価を求める医療従事者や患者による幅広い導入が促進されることが期待されます。
- 2023年2月、フルジェント・ジェネティクスは、常染色体劣性遺伝性疾患およびX連鎖性疾患に関連する787個の遺伝子を検査するBeacon787拡張キャリアスクリーニングパネルを発売しました。この開発により、キャリアスクリーニングの範囲が大幅に拡大され、将来の親に遺伝的リスクプロファイルをより包括的に把握できるようになります。これは、多民族かつ高カバレッジのパネルへの市場トレンドを強化し、臨床および研究室における出生前および妊娠前ケアプログラムにおけるキャリアスクリーニングのより広範な利用を促進するものです。
- 2022年1月、ミテラは、自宅で検査できる生殖遺伝子検査キット「Peaches & Me」と「23 Pears」を米国全50州で提供開始すると発表しました。これらのキットにより、ダウン症候群などの疾患を自宅で検査できるため、アクセス性と利便性が向上します。この開発は、キャリアスクリーニング市場における消費者重視のセグメントの成長を浮き彫りにしており、早期かつ簡便な生殖リスク評価を求める、テクノロジーに精通した健康志向の高い層の間で普及が進むと期待されています。
- 2021年6月、グレイルは50歳以上の成人またはがんリスクの高い人をスクリーニングするために設計された、多がん血液検査「ガレリ」を発売しました。主にがん検出に焦点を当てていますが、この発売は、予防医療における多疾患遺伝子検査と高度なスクリーニング技術への幅広い動きを強調するものです。これは、市場が早期かつ非侵襲的な検出ソリューションと、革新的な遺伝子検査アプローチを日常的な臨床ケアに統合することへと進化していることを示しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。