アジア太平洋地域の前臨床イメージング市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
294.06 Million
USD
451.29 Million
2024
2032
USD
294.06 Million
USD
451.29 Million
2024
2032
| 2025 –2032 | |
| USD 294.06 Million | |
| USD 451.29 Million | |
|
|
|
|
アジア太平洋地域の前臨床イメージング市場セグメンテーション、製品(システムおよびサービス)、試薬(前臨床光学イメージング試薬、前臨床核イメージング試薬、前臨床MRI造影剤、前臨床超音波造影剤、前臨床CT造影剤)、アプリケーション(研究開発、創薬、バイオ流通、がん細胞検出、バイオマーカーなど)、エンドユーザー(契約研究機関、製薬およびバイオテクノロジー企業、学術および政府研究機関、診断センターなど) - 2032年までの業界動向と予測
アジア太平洋地域の前臨床イメージング市場規模
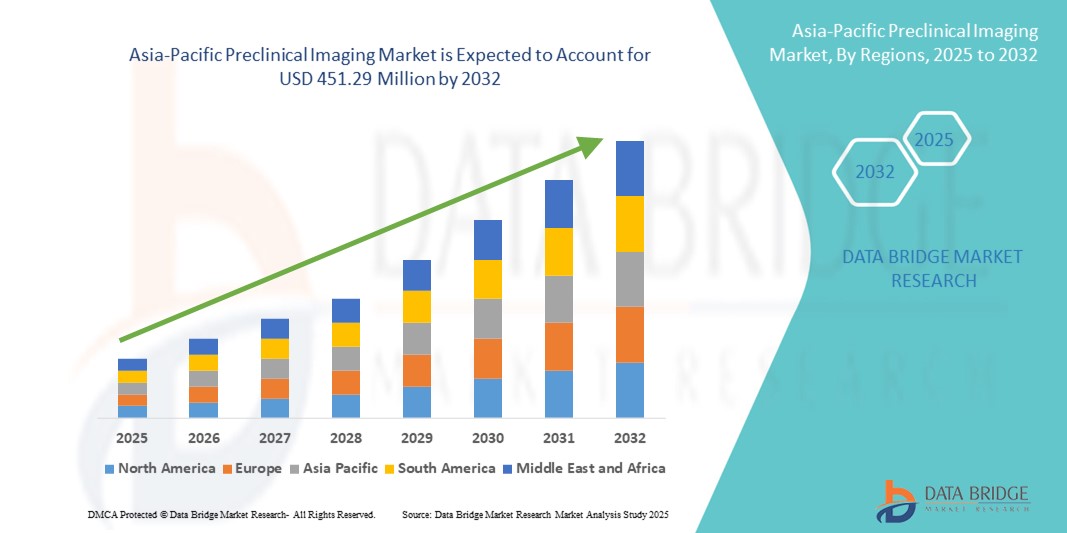
- アジア太平洋地域の前臨床イメージング市場規模は2024年に2億9,406万米ドルと評価され、予測期間中に5.50%のCAGRで成長し、2032年には4億5,129万米ドル に達すると予想されています 。
- 市場の成長は、主に先進的な画像診断法の採用の増加と、地域全体、特に中国、日本、インドなどの国々における前臨床研究および創薬への投資の増加によって推進されています。
- さらに、トランスレーショナルリサーチ、早期疾患診断、非侵襲性イメージング技術への関心の高まりにより、製薬およびバイオテクノロジー研究における前臨床イメージングシステムの活用が促進されています。これらの要因が重なり合い、革新的なイメージングソリューションの普及が加速し、業界の成長を大きく後押ししています。
アジア太平洋地域の前臨床イメージング市場分析
- MRI、 CT、PET 、SPECT、光学イメージングなどの高度なモダリティを網羅する前臨床イメージングは、その非侵襲性、高解像度イメージング、前臨床研究の加速能力により、学術、製薬、バイオテクノロジー研究の現場全体で、創薬、トランスレーショナルリサーチ、早期疾患診断に不可欠な要素になりつつあります。
- 前臨床イメージングの需要の高まりは、主に医薬品研究開発への投資の増加、精密医療への注目の高まり、医薬品開発期間の短縮とトランスレーショナルリサーチの成果の向上を目的とした高度なイメージング技術の採用の増加によって促進されています。
- 中国は、2024年にアジア太平洋地域の前臨床イメージング市場において39%という最大の収益シェアを占め、市場を支配した。その特徴は、バイオメディカル研究に対する多額の政府資金、医薬品製造の急速な拡大、腫瘍学、神経学、バイオマーカー研究における高度なイメージングシステムの導入である。
- インドは、臨床試験の増加、製薬およびバイオテクノロジー研究活動の増加、費用対効果の高いイメージングソリューションの採用の増加により、予測期間中にアジア太平洋地域の前臨床イメージング市場で最も急速に成長する国になると予想されています。
- 前臨床光学イメージング試薬セグメントは、分子イメージング、癌研究、生体内疾患モデリングにおける広範な応用により、2024年に38.7%の市場シェアでアジア太平洋地域の前臨床イメージング市場を支配した。
レポートの範囲とアジア太平洋地域の前臨床イメージング市場のセグメンテーション
|
属性 |
アジア太平洋地域の前臨床イメージング主要市場分析 |
|
対象セグメント |
|
|
対象国 |
アジア太平洋
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
アジア太平洋地域の前臨床イメージング市場動向
マルチモーダルイメージングとAI統合の進歩
- アジア太平洋地域の前臨床イメージング市場において、マルチモーダルイメージングシステムと人工知能(AI)および高度なデータ分析プラットフォームの統合は、重要かつ加速的なトレンドとなっています。この組み合わせにより、画像解像度が向上し、分析が加速し、より正確で予測性の高い前臨床研究が可能になります。
- 例えば、中国ではPET/MRIシステムとAIを活用した画像再構成を組み合わせることで、研究者はより高精度でスキャン時間を短縮した縦断的研究を実施できるようになっています。同様に、日本ではAIを活用した光学イメージングプラットフォームがハイスループットがん研究に導入され、早期発見と治療評価の改善につながっています。
- AIの統合により、画像の自動セグメンテーション、パターン認識、予測モデリングが可能になり、疾患の進行と薬剤の有効性をより深く理解するのに役立ちます。例えば、インドではAIを活用したMRI解析によりバイオマーカーの特定が向上し、手作業による解釈ミスが削減されています。また、マルチモーダルイメージングでは、解剖学的データと機能的データを組み合わせることで包括的な洞察が得られます。
- 集中化された研究室情報管理プラットフォームとイメージングシステムをシームレスに統合することで、研究者は複数の前臨床研究のデータを管理し、ハイスループットのワークフローと再現可能な結果を容易に得ることができます。
- よりインテリジェントで高精度、そして相互接続されたイメージングソリューションへのこのトレンドは、前臨床研究の基準を根本的に変革しつつあります。その結果、MILabsやBrukerなどの企業は、マルチモーダル機能と強化された画像分析機能を備えたAI対応の前臨床イメージングシステムを開発しています。
- AIとマルチモーダル統合を備えた前臨床画像システムの需要は、これらの技術が創薬を加速し、トランスレーショナルリサーチの成果を向上させるため、製薬、バイオテクノロジー、学術研究の各分野で急速に高まっています。
アジア太平洋地域の前臨床イメージング市場の動向
ドライバ
医薬品研究開発とトランスレーショナルリサーチへの投資増加
- アジア太平洋地域、特に中国、日本、インドにおける医薬品およびバイオテクノロジー研究開発への投資の増加は、前臨床イメージング導入の大きな推進力となっています。高度なイメージングシステムは、初期段階の医薬品開発、バイオマーカーの検証、そして疾患モデリングを支援しています。
- 例えば、2024年3月には、中国の主要な研究コンソーシアムが、PET/CTとMRIシステムをAIベースの分析ツールと統合し、腫瘍薬の試験をサポートするために前臨床画像インフラストラクチャを拡張しました。
- 製薬会社が医薬品開発の期間とコストを削減しようとしている中、前臨床イメージングは、疾患の進行と治療効果に関する非侵襲的で高解像度の洞察を提供します。
- 精密医療、早期疾患検出、トランスレーショナルリサーチへの関心が高まるにつれ、学術研究と商業研究の両方の現場で統合された高性能イメージングシステムの需要が高まっています。
- 日本や韓国などの国では、バイオメディカル研究を支援することを目的とした政府の取り組みや助成金により、イメージングインフラへの追加資金が提供され、市場の成長がさらに加速しています。
- 画像機器メーカーと研究機関が協力して特殊な画像ソリューションを共同開発することで、新たな市場機会が生まれています。
抑制/挑戦
高額な設備費と熟練した労働力の必要性
- 高度な前臨床イメージングシステムと試薬の高コストは、特に小規模な研究機関や新興バイオテクノロジー企業にとって、普及への大きな障壁となっています。PET/MRIやマルチモーダル光学イメージングなどのシステムは、多額の設備投資を必要とします。
- さらに、複雑な画像システムの運用には、画像の取得、分析、保守を行うための高度な訓練を受けた人員が必要です。インドや東南アジアなどの新興市場では、熟練した画像専門家の不足が市場浸透を遅らせる可能性があります。
- 例えば、2024年の報告書では、インドのいくつかの中規模バイオテクノロジー研究所が予算の制約と訓練を受けたオペレーターの不足によりPET/CTの導入を遅らせていることが強調されている。
- 一部の企業はより小型でコスト効率の高い画像ソリューションを提供していますが、AI統合型のプレミアムマルチモーダルシステムは依然として高額であり、アクセスが制限されています。
- 特に中国と日本では、前臨床画像診断装置の規制遵守と承認により、導入が遅れ、運用コストが増加する可能性がある。
- 大量の画像データを安全かつ効率的に処理することを含むデータ管理と統合の課題は、ITインフラを持たない小規模機関にとって依然として障壁となっている。
- 研修プログラム、共同研究イニシアチブ、手頃な価格のイメージングプラットフォームの開発を通じてこれらの課題を克服することは、アジア太平洋地域の前臨床イメージング分野の持続的な市場成長にとって極めて重要です。
アジア太平洋地域の前臨床イメージング市場の展望
市場は、製品、試薬、用途、およびエンドユーザーに基づいてセグメント化されています。
- 製品別
製品に基づいて、アジア太平洋地域の前臨床イメージング市場は、システムとサービスに分類されます。システムセグメントは、MRI、CT、PET、SPECT、光学イメージングなどの高度なイメージングモダリティの前臨床研究への導入拡大に牽引され、2024年には65.4%という最大の収益シェアで市場を席巻しました。高解像度イメージングシステムは、製薬会社や学術機関において、詳細な解剖学的・機能的研究を行う上でますます好まれています。システムの信頼性、再現性、長期的な有用性は、創薬やトランスレーショナルリサーチに不可欠な要素となっています。研究機関は、マルチモーダルイメージング研究や縦断的実験をサポートできるシステムを優先しています。さらに、精密医療やバイオマーカー同定への注目の高まりも、この地域におけるイメージングシステムの導入をさらに加速させています。
サービス分野は、専門の前臨床サービスプロバイダーへの画像研究のアウトソーシングの増加に支えられ、2025年から2032年にかけて最も急速な成長を遂げると予想されています。これらのサービスにより、小規模な研究室やCROは、多額の設備投資の負担なしに高度な画像技術を利用できるようになります。前臨床画像サービスプロバイダーは、画像撮影、データ分析、レポート作成を含む包括的なソリューションを提供し、医薬品開発における迅速な意思決定を支援します。この分野は、腫瘍学、神経学、心血管研究における契約ベースの前臨床研究の需要増加の恩恵を受けています。さらに、柔軟なサービスモデルとカスタマイズされた画像ソリューションは、アジア太平洋地域の新興市場における導入を促進しています。これらのサービスは、費用対効果と利便性に優れているため、より幅広いエンドユーザーにとって魅力的なものとなっています。
- 試薬別
試薬に基づいて、アジア太平洋地域の前臨床イメージング市場は、前臨床光イメージング試薬、前臨床核イメージング試薬、前臨床MRI造影剤、前臨床超音波造影剤、および前臨床CT造影剤に分類されます。前臨床光イメージング試薬は、分子イメージング、がん研究、および生体内疾患モデリングにおける広範な応用により、2024年には38.7%のシェアで市場を支配しました。これらの試薬は縦断的研究に広く使用されており、同じ対象を繰り返しイメージングして、疾患の進行または治療反応を監視できます。マルチモーダルイメージングシステムとの互換性により、トランスレーショナルリサーチにおける有用性が向上します。光イメージング試薬はまた、前臨床モデルにおける特定の生体分子および細胞イベントの高感度検出を可能にします。生きた動物における分子相互作用を視覚化する能力は、迅速な医薬品開発とバイオマーカー検証をサポートします。
前臨床MRI造影剤は、軟部組織イメージングおよびバイオマーカー評価におけるMRIの採用増加に牽引され、予測期間中に最も急速な成長を遂げると予想されています。解像度の向上、毒性の低減、そして組織特異的な画像を提供する造影剤の革新が、市場拡大を後押ししています。縦断的研究におけるMRI造影剤の使用増加により、研究者は組織の構造と機能の変化を経時的に追跡することが可能になります。製薬会社は、疾患の進行や治療効果の研究に、高度なMRI造影剤をますます多く使用しています。画像機器メーカーとの共同研究により、次世代MRI造影剤の開発が加速しています。さらに、アジア太平洋地域におけるバイオメディカル研究への政府支援も、前臨床研究におけるこれらの造影剤の採用に貢献しています。
- アプリケーション別
アプリケーションに基づいて、アジア太平洋地域の前臨床イメージング市場は、研究開発、創薬、バイオディストリビューション、がん細胞検出、バイオマーカー、その他に分類されています。研究開発セグメントは、初期段階の創薬とトランスレーショナルリサーチへの投資の増加に支えられ、2024年には44.5%のシェアで市場を支配しました。学術研究や製薬会社の研究開発でイメージングシステムが高く採用されているため、研究者は生体内で疾患モデルを研究し、薬の有効性をリアルタイムで評価できます。前臨床イメージングは詳細なメカニズム研究を容易にし、臨床試験前に薬剤候補を最適化するのに役立ちます。AIと高度な画像解析ソフトウェアの統合により、研究開発ワークフローの効率と精度が向上しています。研究者は、治療標的の検証、薬物動態の分析、毒性の評価にイメージングをますます活用しています。これにより、前臨床イメージングはアジア太平洋地域の現代の生物医学研究の基礎として位置付けられています。
がん細胞検出分野は、がん罹患率の上昇と、抗がん療法の評価における前臨床イメージングの重要性により、2025年から2032年にかけて最も高いCAGR(年平均成長率)を達成すると予想されています。イメージング技術は、動物モデルにおける腫瘍の増殖、転移、治療への反応の追跡を可能にします。分子イメージング剤および光学イメージング剤の使用により、がん細胞を早期段階で正確に可視化することが可能になります。製薬会社は、新規治療薬や免疫療法の試験に前臨床イメージングを活用しています。個別化医療や標的療法への関心の高まりも、需要をさらに押し上げています。さらに、イメージング試薬開発者と研究機関の提携により、高感度がん細胞検出能力が向上しています。
- エンドユーザー別
エンドユーザーに基づいて、アジア太平洋地域の前臨床イメージング市場は、開発業務受託機関(CRO)、製薬・バイオテクノロジー企業、学術・政府研究機関、診断センター、その他に分類されます。製薬・バイオテクノロジー企業は、多額の研究開発費と早期段階の医薬品評価の需要の高まりにより、2024年には47.1%のシェアで市場を支配しました。これらの企業は、候補薬のスクリーニング、毒性評価、バイオマーカーの検証に前臨床イメージングを利用しています。イメージングシステムにより、継続/中止の決定が迅速化され、開発コストと期間が削減されます。自動化されたハイスループットプラットフォームとの統合により、その採用がさらに強化されます。精密医療と標的療法の増加傾向により、前臨床研究におけるイメージング技術への依存が高まっています。イメージング機器や試薬メーカーとのコラボレーションも、このセグメントの拡大を支えています。
学術研究機関および政府研究機関は、生物医学研究への政府資金の増加と研究インフラの拡充に牽引され、予測期間中に最も急速な成長を遂げると予想されています。研究機関は、基礎研究の推進、疾患メカニズムの研究、そして新たな治療戦略の開発のために、前臨床イメージング技術を導入しています。世界的なイメージング機器プロバイダーとの提携により、高解像度システムや最先端の試薬へのアクセスが可能になっています。トレーニングプログラムや共同研究プロジェクトは、学術研究室におけるイメージング技術の幅広い利用を促進しています。マルチモーダルイメージングやAIベースの分析ツールの導入は、研究能力をさらに強化しています。
アジア太平洋地域の前臨床イメージング市場地域分析
- 中国は、2024年にアジア太平洋地域の前臨床イメージング市場において39%という最大の収益シェアを占め、市場を支配した。その特徴は、バイオメディカル研究に対する多額の政府資金、医薬品製造の急速な拡大、腫瘍学、神経学、バイオマーカー研究における高度なイメージングシステムの導入である。
- この地域の研究者や研究機関は、創薬、トランスレーショナルリサーチ、早期疾患検出を加速させる前臨床システムが提供する高解像度の非侵襲性画像化能力を高く評価しています。
- この広範な採用は、製薬およびバイオテクノロジーの研究開発への投資の増加、臨床試験数の増加、精密医療への注目の高まりによってさらに支えられ、前臨床イメージングはアジア太平洋地域の学術研究機関と商業研究機関の両方にとって重要なツールとして確立されています。
中国の前臨床イメージング市場に関する洞察
中国の前臨床イメージング市場は、バイオメディカル研究への政府の巨額な資金提供、医薬品製造の急速な拡大、そして腫瘍学、神経学、バイオマーカー研究におけるマルチモーダルイメージングシステムの導入増加に支えられ、2024年にはアジア太平洋地域で最大の収益シェアを獲得しました。中国はハイテク研究とトランスレーショナルリサーチに基づく医薬品開発に重点を置いており、学術研究室から民間研究室まで、高度なイメージングソリューションの導入を促進しています。
日本の前臨床イメージング市場インサイト
日本の前臨床イメージング市場は、強力な研究開発エコシステム、高い技術導入率、そして精密医療への注力により、勢いを増しています。AIとマルチモーダルイメージングを前臨床研究に統合することで、より正確な疾患モデルの構築と治療評価が可能になり、導入がさらに加速しています。国内の確立された研究インフラと、バイオメディカルイノベーションに対する政府の支援は、市場の成長を継続的に後押ししています。
インドの前臨床イメージング市場に関する洞察
インドの前臨床イメージング市場は、研究インフラの急速な拡大、臨床試験活動の増加、そして費用対効果の高いイメージングシステムの導入増加により、予測期間中にアジア太平洋地域で最も急速な成長を遂げると予想されています。バイオメディカル研究を促進する政府の取り組み、CROの台頭、そして世界的なイメージング技術プロバイダーとの連携は、学術分野と製薬分野の双方で需要を押し上げています。
韓国の前臨床イメージング市場インサイト
韓国の前臨床イメージング市場は、バイオテクノロジー研究開発への投資増加、がん研究および神経学研究における先進的なイメージング技術の導入、そして国内研究機関と世界的なイメージングシステムメーカーとの連携強化に支えられ、着実に拡大しています。同国はデジタルヘルスと革新的なイメージングソリューションに注力しており、市場浸透の拡大を促進しています。
アジア太平洋地域の前臨床イメージング市場シェア
アジア太平洋地域の前臨床イメージング業界は、主に次のような定評ある企業によって牽引されています。
- ブルカー(米国)
- パーキンエルマー(米国)
- FUJIFILM VisualSonics, Inc.(カナダ)
- アスペクトイメージング株式会社(イスラエル)
- トライフォイルイメージング(米国)
- LI-CORバイオサイエンス(米国)
- メディソ株式会社(ハンガリー)
- MILabs BV(オランダ)
- MRソリューションズ株式会社(英国)
- GEヘルスケア(米国)
- シーメンス・ヘルシニアーズAG(ドイツ)
- キヤノンメディカルシステムズ株式会社(日本)
- 上海ユナイテッドイメージングヘルスケア株式会社(中国)
- ミンドレイ・メディカル・インターナショナル・リミテッド(中国)
- 東軟医療システム株式会社(中国)
- 日立メディコ株式会社(日本)
- Koninklijke Philips NV (オランダ)
- オリンパス株式会社(日本)
- 浜松ホトニクス株式会社(日本)
- 3DHISTECH Ltd.(ハンガリー)
アジア太平洋地域の前臨床イメージング市場の最近の動向は何ですか?
- 2024年12月、インタス・ファーマシューティカルズは、コヒーラス・バイオサイエンス社からUDENYCA(ペグフィルグラスチム-cbqv)事業を最大5億5,800万米ドルで買収する契約を締結したことを発表しました。この買収は2025年第1四半期に完了し、バイオシミラー医薬品および関連資産すべてが含まれます。UDENYCAは、化学療法誘発性好中球減少症の治療薬であるニューラスタのバイオシミラーです。この戦略的買収により、インタスのバイオシミラーポートフォリオが強化され、ペグフィルグラスチムの主要グローバルサプライヤーとしての地位が確固たるものになります。
- 2024年10月、世界保健機関(WHO)はインドのニューデリーで医薬品安全性監視パートナー会議を開催しました。第19回国際医薬品規制当局会議(ICDRA)の期間中に開催されたこの会議には、68カ国の規制当局が参加し、WHOグローバル・スマート医薬品安全性監視戦略の草案を検討しました。この取り組みの目標は、加盟国間の医薬品安全性監視活動の収束的進化を促進し、調和のとれた実用的な規制要件の策定につなげることです。
- 2024年4月、WuXi AppTecの子会社であるWuXi STAは、中国・泰興市に169エーカーの新たな医薬品有効成分(API)製造施設を建設するなど、複数の拠点を擁するグローバルな拡張計画を発表しました。この拡張は、他の施設と併せて、アジア太平洋地域および世界における前臨床試験を含む医薬品開発サービスの需要の高まりに対応するための製造能力と生産能力の強化を目指しています。
- 2022年11月、ブルカー・コーポレーションは、自由に動く動物の脳画像撮影を目的とした小型顕微鏡(「ミニスコープ」)を専門とする神経科学企業、インスコピックス社の買収を発表しました。この買収により、神経疾患の理解に不可欠な動物の神経ネットワーク機能のより深い探求を可能にする製品とサービスが加わり、ブルカーの神経科学ポートフォリオが強化されます。
- FUJIFILM VisualSonics Inc.は、2022年5月、世界初となる超高周波から低周波(71MHz~1MHz)までをカバーする前臨床用超音波・光音響イメージングシステム「Vevo F2」を発売しました。本システムは、HD画像処理技術と、トランスデューサーからディスプレイまでの全く新しい信号経路を採用し、より鮮明な画像と高いフレームレートを実現しています。この進歩は、腫瘍学、発生生物学、神経生物学、心臓病学など、多岐にわたる生物学・生理学研究に特に適しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。