アジア太平洋地域の精神科/うつ病における薬理遺伝学検査市場、タイプ別(不安、気分障害、うつ病、双極性障害、精神病性障害、摂食障害)、検査タイプ別(全ゲノム配列解析、染色体アレイベースの検査)、患者タイプ別(小児、成人、高齢者)、遺伝子タイプ別(CYP2C19、CYP2C9、VKORC1、CYP2D6、HLA-B、HTR2A/C、HLA-A、CYP3A4、SLC6A4、MTHFR、COMT、その他)、製品別(機器、消耗品、ソフトウェア&サービス)、エンドユーザー別(病院、診療所、診断研究所、学術研究機関、その他)、流通チャネル別(直接入札、第三者流通病院薬局、その他)– 2029年までの業界動向と予測。
アジア太平洋地域の精神医学/うつ病における薬理遺伝学検査市場の分析と洞察
薬理遺伝子検査は、人が薬剤をどのように代謝するかに関する情報を提供することで、医療専門家の役に立ちます。この情報は、医師やその他の関係者が望ましくない結果をもたらす可能性のある抗うつ薬の処方を避けるのに役立ちます。薬理遺伝子学は、大うつ病性障害 (MDD) の治療における抗うつ薬の反応と忍容性を予測する上で有望であることが示されています。薬理遺伝子学は、抗うつ薬の選択と投与量を導くことで臨床結果を改善できます。バイオテクノロジー分野の成長と医療費の増加により、精神医学/うつ病における薬理遺伝子検査の需要が加速しています。

癌の罹患率の増加、うつ病やその他の精神疾患の治療における新技術により、精神科/うつ病の機器や処置における薬理遺伝学検査の採用が増加しており、非外科的処置に対する好みの高まりが、予測期間中の市場の需要を押し上げる主な要因となっています。ただし、検査に関連する高コスト、厳格な規制、認識不足により、予測期間中の精神科/うつ病の薬理遺伝学検査市場の成長が妨げられる可能性があります。
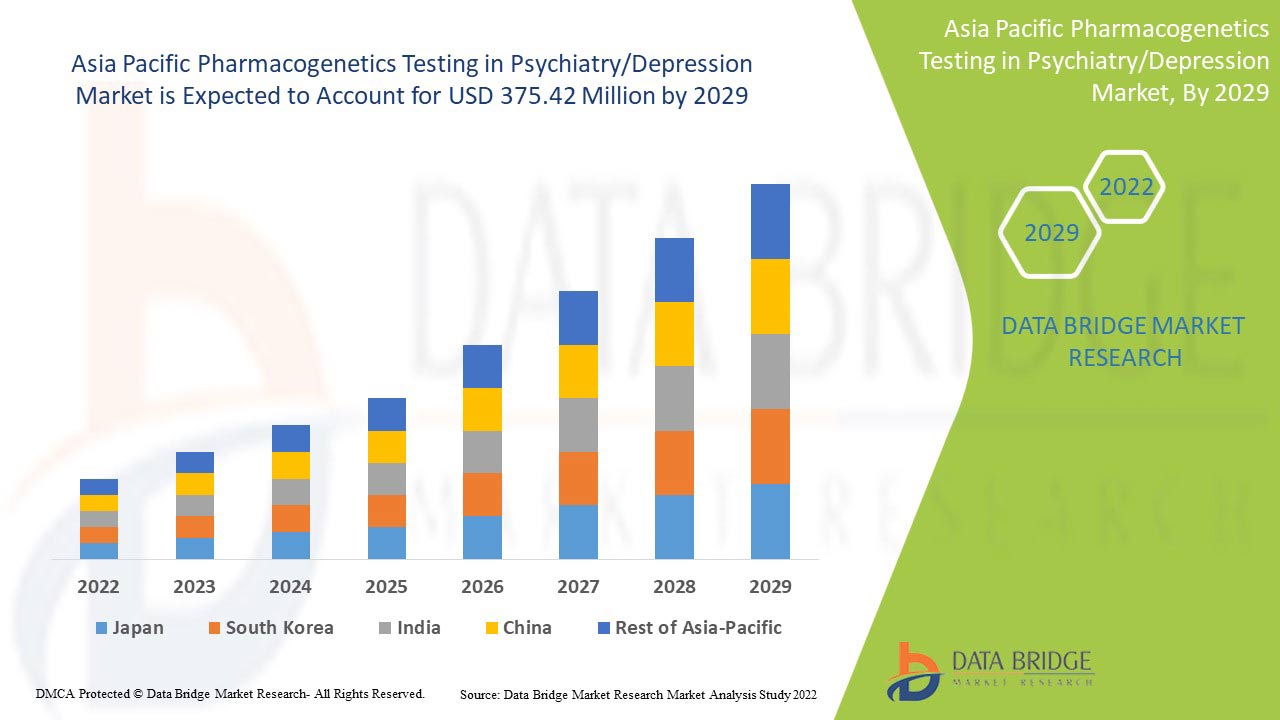
データブリッジ市場調査は、アジア太平洋地域の精神医学/うつ病における薬物遺伝学検査市場は、予測期間中に10.6%のCAGRで成長し、2029年までに3億7,542万米ドルに達すると予測しています。アジア太平洋地域の人口におけるうつ病率の上昇により、不安障害が市場で最大のタイプセグメントを占めています。この市場レポートでは、価格分析、特許分析、技術進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 |
|
定量単位 |
売上高(百万米ドル)、販売数量(個数)、価格(米ドル) |
|
対象セグメント |
アジア太平洋地域の精神科/うつ病における薬理遺伝学検査市場、用途別(新薬候補、薬剤の最適化と転用、前臨床試験と承認、薬剤モニタリング、新しい疾患関連ターゲットと経路の発見、疾患メカニズムの理解、情報の集約と統合、仮説の形成と適格性、新規薬剤設計、旧薬の薬剤ターゲットの発見など)、技術別(機械学習、ディープラーニング、自然言語処理など)、薬剤タイプ別(小分子、大分子)、提供内容別(ソフトウェア、サービス)、適応症別(免疫腫瘍学、神経変性疾患、心血管疾患、代謝性疾患など)、最終用途別(開発業務受託機関(CRO)、製薬・バイオテクノロジー企業、研究センター、学術機関など) |
|
対象国 |
中国、日本、インド、韓国、シンガポール、タイ、マレーシア、オーストラリア、ベトナム、フィリピン、インドネシア、その他のアジア太平洋諸国 |
|
対象となる市場プレーヤー |
- Genelex(Invite corporationの一部)、Genewiz(Azenta Life Sciencesの一部)、MD Labs、BiogeneiQ、Inc.、ONEOME、LLC、Myriad Genetics、Inc.、GenXys、Castle Biosciences、Inc.、PacBio、QIAGEN、Thermo Fisher Scientific Inc.、AB-Biotics、SA、Coriell Life Sciences、Eurofins Scientific、Illumina、Inc.、Dynamic DNA Laboratories、STADAPHARM GmbH、Color、cnsdose、Genomind、Inc.、Healthspek、myDNA Life Australia Pty Ltd.、HudsonAlpha、Sonic Healthcare Limitedなど。 |
アジア太平洋地域の精神医学/うつ病における薬理遺伝学検査市場の定義
薬理ゲノム検査は最近、拡張可能になり、大うつ病性障害 (MDD) の診断に利用できるようになりました。臨床医は、薬理ゲノム (PGx) 検査を精神疾患の投薬決定を導くための必須ツールとしてますます認識しています。予測期間中、PGx 検査の広範な導入が市場を牽引します。
個別化医療、階層化医療、精密医療という用語は薬理遺伝学と近い関係にありますが、これらはより広い用語であり、追加の非遺伝的要因もカバーしています。それでも、薬理遺伝学はこれらの分野の重要な要素です。薬理遺伝学は主にヒトの生殖細胞系列 DNA の変異に関係していますが、最近では気分障害や精神疾患の理解にも進歩が見られます。
薬理遺伝学検査は、薬物と人の遺伝子反応の相互作用を研究し、薬物の効果に影響を与える遺伝子変異を探します。多くの研究者や科学者が薬物と個々の遺伝子の独特な相互作用を特定し、カスタマイズされた医薬品や個人向け医薬品の開発に使用できる貴重な洞察を提供しているため、この検査の需要は高まっています。
アジア太平洋地域の精神医学/うつ病における薬理遺伝学検査市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 精神疾患およびうつ病患者数の増加
うつ病は世界中でよく見られる病気で、人口の約 3.8% が罹患しており、成人では 5.0%、60 歳以上の成人では 5.7% に上ります。うつ病は軽度から重度まで深刻な健康状態になる可能性があり、患者に大きな苦しみを与え、最悪の場合には自殺につながることもあります。45 種類以上の抗うつ薬が利用可能ですが、最適ではない反応が課題であり、遺伝的変異、精神医学/うつ病の結果であると考えられています。長期にわたるうつ病エピソードの重症度とパターンに応じて、医療提供者は行動活性化、認知行動療法、対人関係療法などの心理学的診断、および/または選択的セロトニン再取り込み阻害薬 (SSRI) や三環系抗うつ薬 (TCA) などの抗うつ薬を提供する場合があります。この種の精神障害にはさまざまな薬剤が使用されます。
うつ病の罹患率が上昇するにつれ、遺伝子変異の影響を研究して個別診断を提供することを目的とする薬理遺伝学検査の需要も高まっています。市場は成長期に成長すると予想されています。
- 個別化医療と精密医療の需要増加
薬理遺伝学検査は、薬の効果に影響を与える可能性のある遺伝子変異を検索するため、医療専門家が患者に最適な薬を選択するのに役立ちます。
医療はパーソナル化しており、患者は次第にパーソナライズされた医薬品による治療結果の改善と副作用の軽減に関心を示すようになっています。パーソナライズされた医療は、高い安全域と最良の反応で治療をカスタマイズできる可能性があります。この傾向は、主にゲノム配列の改良によって推進されています。
パーソナルヘルスケアへの移行は、医薬品製造の変化を意味します。製造業者は、小分子の作成から小分子と遺伝子治療の組み合わせへと移行しています。スポンサーは、非効率的な大規模バッチ生産を新しい技術への投資に置き換え、パーソナライズされた医薬品を製造することに重点を置いています。
拘束
- 熟練した医療およびゲノム専門家の不足
ほとんどの臨床医は依然として薬理遺伝学 (PGx) 検査とそれに続くデータ解釈に自信がなく、この分野に関する知識が不十分であることを示しています。薬理遺伝学 (PGx) 検査の理解に関する専門知識に関して、医療従事者のリテラシーを向上させる必要性が強調されています。
薬理遺伝学の可能性に関する医師の認識不足や、検査結果の説明不足も、患者向けのパーソナライゼーション技術を低下させます。医科大学でのテーマ別トレーニングコースの開発に加えて、継続的な専門教育システムでの教育サイクルや、学術インターネットポータル、ウェビナーなど、開業医向けの情報の無料提供も必要です。臨床薬理学者は、薬理遺伝学検査の実施において重要な役割を果たします。
薬理遺伝学の分野における臨床薬理学者の能力は非常に重要です。臨床薬理学者は、臨床診療における遺伝子型判定の応用を組織し、検査を解釈し、特定の病態を持つ患者に対する薬理遺伝学の可能性について医師に知らせる人物であり、薬理遺伝学の導入プロセスにおいて科学界、医療制度、開業医の間の主要なつながりとして機能します。
機会
-
テクノロジーの進歩
薬理ゲノム学の進歩により、精神疾患の臨床診療に個別化医療を取り入れる機会が増えています。個別化医療は、最適な個人の医療決定を達成する方法で疾患を予防、診断、治療、および監視する包括的で前向きなアプローチと定義できます。現在、100 種類を超える医薬品に、医療技術の進歩により適用可能な可能性のある薬理ゲノムバイオマーカーに関する米国食品医薬品局 (FDA) ラベルが付いています。また、うつ病様疾患の薬理遺伝子検査を促進するための新しい高度な方法が開発されています。これらの検査では、治療計画を策定するための正確な結果を得るために高度な遺伝子検査方法を使用します。検査をサポートする技術の向上により検査オプションのアクセシビリティが向上し、この情報が利用可能になったときにそれをどのように使用するかを臨床医が理解するのに役立つリソースの数が増えていることで、個別化医療または精密医療のこの側面が現実のものになっています。したがって、医療提供者は薬理ゲノム検査の科学的および臨床的関連性をより意識する必要があります。
この検査は、薬と個人の遺伝子構成との関連性を明らかにするのにも役立ちます。これは、重度のうつ病やその他の精神疾患の治療のために患者に投与する薬を決定するのに役立ちます。
チャレンジ
- 新製品や機器の承認に関する政府の厳しい規制
製品の有効性と安全性に関する懸念から、ほとんどの政府は、新しい医療製品や検査の開発を監視するための規制機関や政策を策定しています。これらの医療製品は、製品が安全で、十分に研究され、副作用がないことを保証する厳格な規制基準に合格した後に使用できるようになります。
The recent guidelines and the amendment have adequate guidance for manufacturers. International regulations such as food, drug, and administration play a major role in the new launch of the medical product or test into the market. Thus, it can be a major restraint for the market. Therefore, strict government regulation on new products and instrument approval will likely impact the market.
Post-COVID-19 Impact on Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market
The COVID-19 outbreak had a beneficial impact on the expansion of the pharmacogenetics testing industry. The pandemic has had a negative impact on the pharmacogenomics market growth on account of the temporary halt in research activities in this field, coupled with the low influx of patients in hospitals and diagnostic centers. Since the second half of 2020, with the rising demand for research on certain drugs and testing kits for COVID-19, pharmacogenomic practices have been in vogue.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities, product launches, and strategic partnerships to improve the technology and test results involved in the pharmacogenetics testing market.
Recent Developments
- In April 2022, Blue Care Network (BCN) launched a precision medicine program. Blue cross personalized medicine leverages pharmacogenomics, or genetic testing, to personalize and tailor medication treatments more effectively for select members based on a review of their prescribed medications for various diagnosis, including behavioral health, cardiology, cardiovascular, and oncology. OneOme LLC has helped BCN achieve its precision medicine program goals and reduce the total cost of care, and improve patient health outcomes by reducing adverse drug reactions. This has helped the company to enhance its product portfolio.
- In February 2022, PacBio, a leading provider of high-quality, highly accurate sequencing platforms, announced that it is supporting The Hospital for Sick Children (SickKids) in Toronto, Canada, in using HiFi whole genome sequencing (HiFi WGS) to potentially identify genetic variants that may be associated with medical and developmental conditions. Samples that are examined using HiFi WGS were previously sequenced using short-read DNA sequencing technology but still lack the identification of a disease-causing variant. This has helped the company to enhance the use of its products.
- In July 2022, according to a new nationwide study of nearly 2,000 veterans conducted by the U.S. Department of Veterans Affairs (VA), and Major Depressive Disorder (MDD) remission rates were significantly improved when clinicians had access to GeneSight Psychotropic test results from Myriad Genetics, Inc. in largest ever mental health PGx randomized controlled trial. This has helped the company to show its progress in pharmacogenetic testing.
- In January 2021, myDNA Life Australia Pty Ltd announced a merger with the U.S., Houston-based consumer DNA test company, FamilyTreeDNA, and its parent company. Gene by Gene for revolutionizing the field of pharmacogenomics, making truly personalized medicine a reality, before expanding into nutrigenomics to deliver actionable, personalized nutrition, fitness, and skincare recommendations. This has helped the company to expand its business.
Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market Scope
Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into type, test type, gene type, patient type, product, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Type
- Anxiety
- Mood Disorders
- Depression
- Bipolar Disorders
- Psychotic Disorders
- Eating Disorders
On the basis of type, Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into anxiety, mood disorders, depression, bipolar disorders, psychotic disorders, and eating disorders.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Test Type
- Whole Genome Sequencing
- Chromosomal Array-Based Tests
On the basis of test type, Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into whole genome sequencing, and chromosomal array-based tests.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Gene Type
- CYP2C19
- CYP2C9 AND VKORC1
- CYP2D6
- HLA-B
- HTR2A/C
- HLA-A
- CYP3A4
- SLC6A4
- MTHFR
- COMT
- OTHERS
On the basis of gene type, Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT, and others.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Patient Type
- Child
- Adult
- Geriatric
On the basis of patient type, the Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into child, adult, and geriatric.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Product
- Instruments
- Consumables
- Software & Services
On the basis of product type, Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into instruments, consumables, and software & services.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By End User
- Hospitals & Clinics
- Dignostics Laboratories
- Academic And Research Institutes
- Others
On the basis of end user, Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into hospitals and clinics, diagnostics laboratories, academic and research institutes, and others.
- Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market, By Distribution Channel
- Direct Tender
- Third-Party Distribution
- Hospital Pharmacy
- Others

On the basis of distribution channel, the Asia Pacific pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third-party distribution hospital pharmacies, and others.
Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis/Insights
The Asia Pacific pharmacogenetics testing in psychiatry/depression market is analyzed, and market size information is provided by the type, test type, gene type, patient type, product, end user, and distribution channel.
The countries covered in this market report are China, Japan, India, South Korea, Singapore, Thailand, Malaysia, Australia, Vietnam, Philippines, Indonesia, and rest of the Asia-Pacific.
In 2022, Asia Pacific is the third most dominating due to the rising in strategic collaborations and launches by the key market players. Japan is expected to grow due to the rise in technological advancement in pharmacogenetics testing.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Asia Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Asia Pacific Pharmacogenetics Testing in Psychiatry/Depression Market Share Analysis
Asia Pacific pharmacogenetics testing in psychiatry/depression market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Asia Pacific pharmacogenetics testing in psychiatry/depression market.
Some of the major players operating in the Asia Pacific pharmacogenetics testing in psychiatry/depression market are
- Genelex (Part of Invitae corporation)
- Genewiz (Part of Azenta Life Sciences)
- MD Labs
- BiogeneiQ, Inc.
- ONEOME, LLC
- Myriad Genetics, Inc.
- GenXys
- Castle Biosciences, Inc.
- PacBio
- QIAGEN
- Thermo Fisher Scientific Inc.
- AB-Biotics, S.A.
- Coriell Life Sciences
- Eurofins Scientific
- Illumina, Inc.
- Dynamic DNA Laboratories
- STADAPHARM GmbH
- Color
- Cnsdose
- Genomind, Inc.
- Healthspek
- myDNA Life Australia Pty Ltd.
- HudsonAlpha
- ソニックヘルスケア株式会社
調査方法: アジア太平洋地域の精神医学/うつ病市場における薬理遺伝学検査
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。さらに、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、アジア太平洋と地域、ベンダー シェア分析が含まれます。さらに問い合わせる場合は、アナリストへの電話をリクエストしてください。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
- introduction
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- OVERVIEW of Asia-Pacific pharmacogenetic testing in psychiatry/depression market
- LIMITATIONs
- MARKETS COVERED
- MARKET SEGMENTATION
- MARKETS COVERED
- geographical scope
- years considered for the study
- currency and pricing
- DBMR TRIPOD DATA VALIDATION MODEL
- MULTIVARIATE MODELLING
- type LIFELINE CURVE
- primary interviews with key opinion leaders
- DBMR MARKET POSITION GRID
- market application coverage grid
- vendor share analysis
- secondary sourcEs
- assumptions
- EXECUTIVE SUMMARY
- premium insights
- REGULATIONS: ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- AUSTRALIA
- SOUTH KOREA
- market overview
- drivers
- RISING NUMBER OF POPULATION SUFFERING FROM DEPRESSIVE DISORDER
- INITIATIVES TAKEN BY MANUFACTURERS
- GROWING BIOTECHNOLOGY SECTOR ALONG WITH RISING HEALTHCARE EXPENDITURE
- INCREASING INTEREST FOR PERSONALIZED AND PRECISION MEDICATION
- GROWING MEDICAL TOURISM
- RESTRAINTS
- LACK OF STRONG CLINICAL EVIDENCE
- HIGH COST
- LACK OF REIMBURSEMENT
- OPPORTUNITIES
- TECHNOLOGICAL ADVANCEMENTS
- EMERGENCE OF NEW PLAYERS
- Untapped market
- CHALLENGES
- STRINGENT GOVERNMENT REGULATION
- SHORTAGE OF SKILLED PERSONNEL
- COVID-19 IMPACT ON ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- IMPACT ON PRICE
- IMPACT ON DEMAND
- IMPACT ON SUPPLY
- KEY INITIATIVES BY MARKET PLAYER DURING COVID 19
- CONCLUSION:
- Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
- overview
- WHOLE GENOME SEQUENCING
- array-based TESTS
- Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES
- overview
- cyp2c19
- CYP2C9 and VKORC1
- cyp2d6
- HLA-B
- htr2a/c
- HLA-A
- cyp3A4
- slc6a4
- MTHFR
- COMT
- others
- Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE
- overview
- PRESCRIPTION DRUGS
- Over-the-counter medications
- RECREATIONAL DRUGS
- VITAMINS/NUTRACEUTICals
- ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE
- overview
- SALIVA
- BLOOD
- ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY application
- overview
- DRUG DEVELOPMENT
- CLINICAL PRACTICE
- Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER
- overview
- pharmaceutical and biotechnology companies
- HEALTHCARE PROVIDERS
- research centers and academic INSTITUTES
- others
- Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
- overview
- RETAIL PHARMACIES
- HOSPITAL PHARMACIES
- MAIL-ORDER PHARMACIES
- DIRECt-to-customer services
- ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, by Country
- overview
- Asia-Pacific
- JAPAN
- cHINA
- SOUTH KOREA
- INDIA
- AUSTRALIA
- SINGAPORE
- THAILAND
- MALAYSIA
- indonesia
- philipPines
- rest of asia-pacific
- Asia-Pacific pharmacogenetic testing in psychiatry/depression market: COMPANY landscape
- company share analysis: Asia-Pacific
- swot analysis
- company profile
- MYRIAD GENETICS, INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- THERMO FISHER SCIENTIFIC INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- STADA ARZNEIMITTEL AG
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- sonic healthcare
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- Illumina, Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- AB-Biotics, S.A.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- 6.3 PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- ALTHEADX
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- cnsdose
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- luminex corporation
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- mydna life australia pty ltd.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PerkinElmer Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- R-Biopharm AG
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- questionnaire
- related reports
表のリスト
TABLE 1 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 2 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2019-2028 (USD MILLION)
TABLE 3 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 4 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE, 2019-2028 (USD MILLION)
TABLE 5 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 6 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 7 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 8 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2019-2028 (USD MILLION)
TABLE 9 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 10 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 11 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 12 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 13 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 14 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 15 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 16 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 17 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 18 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 19 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 20 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 21 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 22 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 23 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 24 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 25 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 26 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 27 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 28 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 29 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 30 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 31 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 32 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 33 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 34 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 35 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 36 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 37 India PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 38 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 39 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 40 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 41 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 42 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 43 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 44 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 45 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 46 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 47 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 48 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 49 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 50 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 51 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 52 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 53 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 54 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 55 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 56 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 57 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 58 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 59 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 60 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 61 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 62 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 63 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 64 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 65 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 66 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 67 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 68 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 69 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 70 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 71 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 72 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 73 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 74 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 75 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 76 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 77 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 78 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 79 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
TABLE 80 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2021-2028 (USD MILLION)
TABLE 81 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD million)
TABLE 82 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD million)
TABLE 83 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD million)
TABLE 84 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD million)
TABLE 85 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD million)
TABLE 86 Rest of Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD million)
図表一覧
FIGURE 1 Asia-Pacific PHARMACOGENETIC TESTIG IN PSYCHIATRY/DEPRESSION market: segmentation
FIGURE 2 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: data triangulation
FIGURE 3 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: DROC ANALYSIS
FIGURE 4 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: Region vs country MARKET ANALYSIS
FIGURE 5 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: COMPANY RESEARCH ANALYSIS
FIGURE 6 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: DBMR MARKET POSITION GRID
FIGURE 7 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: MARKET APPLICATION COVERAGE GRID
FIGURE 8 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: vendor share analysis
FIGURE 9 Asia-Pacific pharmacogenetic testing in psychiatry/depression market: SEGMENTATION
FIGURE 10 initiatives taken by manufacturers is expected to drive THE Asia-Pacific pharmacogenetic testing in psychiatry/depression market IN THE FORECAST PERIOD of 2021 to 2028
FIGURE 11 whole genome sequencing segment is expected to account for the largest share of the Asia-Pacific pharmacogenetic testing in psychiatry/depression market in 2021 & 2028
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
FIGURE 13 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2020
FIGURE 14 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021-2028 (USD MILLION)
FIGURE 15 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 16 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 17 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2020
FIGURE 18 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2021-2028 (USD MILLION)
FIGURE 19 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, CAGR (2021-2028)
FIGURE 20 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, LIFELINE CURVE
FIGURE 21 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2020
FIGURE 22 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2021-2028 (USD MILLION)
FIGURE 23 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 24 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 25 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2020
FIGURE 26 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2021-2028 (USD MILLION)
FIGURE 27 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 28 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, LIFELINE CURVE
FIGURE 29 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2020
FIGURE 30 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2021-2028 (USD MILLION)
FIGURE 31 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 32 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2020
FIGURE 34 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2021-2028 (USD MILLION)
FIGURE 35 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, CAGR (2021-2028)
FIGURE 36 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 38 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021-2028 (USD MILLION)
FIGURE 39 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 40 Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 42 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020-2028)
FIGURE 43 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 44 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 45 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 46 Asia-Pacific pharmacogenetic testing in psychiatry/DEPRESSION MARKET: company share 2020 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。













