臨床試験は、ワクチンやその他の医薬品の開発と試験に使用されます。彼らは、新型コロナウイルス感染症のパンデミックを封じ込める上で極めて重要でした。たとえば、エアランゲン大学ニュルンベルク医科大学は、SARS-CoV-2感染歴が確認された小児および青少年における肺骨格変化の発生率を調べる臨床試験を2021年8月に開始した(LF-MRI)。新型コロナウイルス感染症(COVID-19)の場合、ワクチンや治療法の開発には臨床試験が不可欠だ。たとえば、ファイザー・ビオンテックとモデルナのワクチンはどちらも臨床試験を通じて開発された。これらのワクチンは安全で、新型コロナウイルス感染症(COVID-19)の予防に効果的であることが示されています。
完全なレポートにアクセスするには:https://www.databridgemarketresearch.com/reports/global-clinical-trial-imaging-market
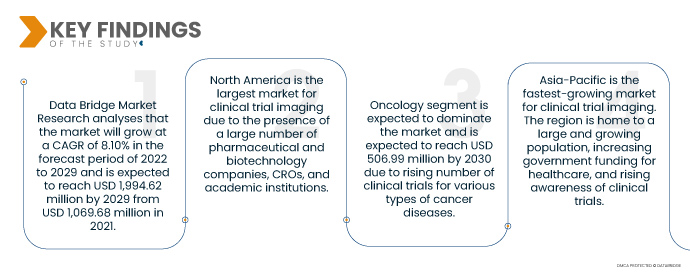
データブリッジ市場調査は次のように分析しています。 治験画像市場 2022年から2029年の予測期間に8.10%のCAGRで成長し、2021年の10億6,968万米ドルから2029年までに19億9,462万米ドルに達すると予想されています。製薬企業やバイオテクノロジー企業は、新薬を開発するために研究開発(R&D)に多額の投資を行っています。薬と治療法。これにより、新しい治療法の安全性と有効性を評価するために使用される臨床試験画像処理の需要が増加しています。製薬会社やバイオテクノロジー会社は臨床試験を CRO にアウトソーシングするケースが増えています。これは、臨床試験の実施にかかるコストと複雑さが原因です。 CRO は、臨床試験の画像処理など、臨床試験を管理するための専門知識とリソースを備えています。
慢性疾患の増加は 市場の成長率を牽引すると予想される
世界的に人口は高齢化しており、これによりがん、心血管疾患、アルツハイマー病などの慢性疾患の有病率が増加しています。これらの疾患では、診断、治療計画、治療反応のモニタリングのために画像処理が必要になることがよくあります。近年、画像技術が大幅に進歩し、臨床試験画像の精度と効率が向上しました。たとえば、PET や MRI などの新しい画像技術は、従来の X 線や超音波よりも詳細な身体の画像を提供できます。これにより市場の成長が促進されます。
レポートの範囲と市場セグメンテーション
|
レポート指標
|
詳細
|
|
予測期間
|
2022年から2029年
|
|
基準年
|
2021年
|
|
歴史的な年
|
2020 (2014 ~ 2019 にカスタマイズ可能)
|
|
量的単位
|
収益(百万米ドル)、数量(単位)、価格(米ドル)
|
|
対象セグメント
|
製品とサービス (サービスとソフトウェア)、モダリティ (コンピューター断層撮影、磁気共鳴画像法、心エコー検査、核医学、陽電子放出断層撮影、X 線、超音波、光干渉断層撮影など)、アプリケーション (腫瘍学、神経学、内分泌学、心臓学、皮膚科、血液内科など)、エンドユーザー(製薬・バイオテクノロジー企業、受託研究機関、医療機器メーカー、学術・政府系研究機関など)、販売代理店(直販、入札販売)
|
|
対象国
|
北米の米国、カナダ、メキシコ、ドイツ、フランス、英国、オランダ、スイス、ベルギー、ロシア、イタリア、スペイン、トルコ、その他の欧州諸国、中国、日本、インド、韓国、シンガポール、マレーシア、オーストラリア、タイ、インドネシア、フィリピン、アジア太平洋地域(APAC)内のその他のアジア太平洋地域(APAC)、サウジアラビア、UAE、南アフリカ、エジプト、イスラエル、中東の一部としての中東およびアフリカ地域(MEA)のその他の地域およびアフリカ (MEA)、ブラジル、アルゼンチン、南アメリカのその他の地域 (南アメリカの一部)
|
|
対象となる市場プレーヤー
|
Navitas Life Sciences (インド)、Resonance Health Analytical Services (オーストラリア)、BioTelemetry (米国)、a Philips Company (オランダ)、IXICO plc (英国)、ICON plc (アイルランド)、Image Core Lab (インド)、アナグラム 4 臨床試験(スペイン)、Quotient Sciences(英国)、Radiant Sage LLC(インド)、WORLDCARE CLINICAL(米国)、Clario(米国)、Paraxel International Corporation(米国)、Median Technologies(フランス)、Perspectum(英国)、Calyx(英国)
|
|
レポートで取り上げるデータポイント
|
データブリッジ市場調査チームが厳選した市場レポートには、市場価値、成長率、市場セグメント、地理的範囲、市場プレーヤー、市場シナリオなどの市場洞察に加えて、詳細な専門家分析、患者疫学、パイプライン分析が含まれています。 、価格分析、および規制の枠組み
|
セグメント分析:
世界の臨床試験イメージング市場は、製品とサービス、モダリティ、アプリケーション、エンドユーザー、およびディストリビューターに基づいて、5 つの主要なセグメントに分類されます。
- 製品とサービスに基づいて、市場はサービスとソフトウェアに分類されます。
サービス部門が臨床試験画像市場を支配すると予想されている
サービス部門は市場を支配すると予想されており、企業が新分子を発売するのに有益な特定の臨床試験に関する正確なデータとコンサルティングを製薬会社やバイオテクノロジー企業に提供するため、2030年までに7億6,338万米ドルに達すると予想されている。
- モダリティに基づいて、市場はコンピューター断層撮影、磁気共鳴画像法、超音波、陽電子放出断層撮影、X線、心エコー検査、核医学、光干渉断層撮影などに分類されます。
コンピュータ断層撮影分野が臨床試験画像市場を支配すると予想されている
コンピュータ断層撮影セグメントは市場を支配すると予想されており、慢性疾患の増加により2030年までに2億6,237万4,000米ドルに達すると予想されています。
- アプリケーションに基づいて、市場は心臓病学、神経学、内分泌学、腫瘍学、皮膚科、血液学などに分類されます。腫瘍学分野は市場を支配すると予想されており、さまざまな種類のがん疾患に対する臨床試験数の増加により、2030年までに5億699万米ドルに達すると予想されています。
- エンドユーザーに基づいて、市場はバイオテクノロジー企業、医療機器メーカー、製薬会社、受託研究機関、学術および政府研究機関などに分類されます。製薬およびバイオテクノロジー企業セグメントが市場を支配すると予想されており、臨床試験中のパイプライン医薬品の数が増加しているため、2030年までに2億798万米ドルに達すると予想されています。
- 販売代理店に基づいて、市場は直接販売と入札販売に分類されます。直接販売セグメントは市場を支配すると予想されており、直接販売はエンドユーザーにコストメリットをもたらすため、2030年までに6億731万2000米ドルに達すると予想されています。
主要プレーヤー
Data Bridge Market Research は、次の企業を主要な市場プレーヤーとして認識しています: Navitas Life Sciences (インド)、Resonance Health Analytical Services (オーストラリア)、BioTelemetry (米国)、a Philips Company (オランダ)、IXICO plc (英国)、ICON plc (アイルランド)、Image Core Lab (インド)、anagram 4 clinical trials (スペイン)、Quotient Sciences (英国)、Radiant Sage LLC (インド)、WORLDCARE CLINICAL (米国)、Clario (米国)、Paraxel International Corporation (米国)、Median Technologies (フランス)、Perspectum (英国)、Calyx (英国)。
市場開拓
- 2022年、ブルカーは5月に開催された国際磁気共鳴医学会(ISMRM)2022会議で、市場をリードする同社の前臨床磁気共鳴画像法(MRI)システム製品向けに革新的な7テスラおよび9.4テスラの伝導冷却マクスウェル磁石を発表した。
- 2022年に向けて、富士フイルムインドは3月にインドでCT、MRI、超音波装置の新ラインを発表した。最新のポートフォリオには、コンピューター断層撮影 (CT)、磁気共鳴画像法 (MR)I、X 線、人工知能、内視鏡検査、Scenaria View、Supria シリーズ、The Echelon Smart、そしてアリエッタシリーズ。
- 2020年、ICON plcは2月に欧州の医療機器CRO、償還、規制コンサルタントであるMedPass Internationalを買収した。この買収は、ICON の医療機器および診断研究サービスのヨーロッパ全土への拡大に貢献したと言われています。
地域分析
地理的に、市場レポートの対象国は、北米では米国、カナダ、メキシコ、ドイツ、フランス、英国、オランダ、スイス、ベルギー、ロシア、イタリア、スペイン、トルコ、欧州のその他の地域、中国、日本、インドです。 、韓国、シンガポール、マレーシア、オーストラリア、タイ、インドネシア、フィリピン、アジア太平洋地域(APAC)のその他の地域(APAC)、サウジアラビア、UAE、南アフリカ、エジプト、イスラエル、中東のその他の地域、および中東の一部としてのアフリカ (MEA)、および南アメリカの一部としてのアフリカ (MEA)、ブラジル、アルゼンチン、および南アメリカのその他の地域。
データブリッジ市場調査分析によると:
北米は2022年から2029年の予測期間中に臨床試験画像市場で支配的な地域です
北米は、多数の製薬企業、バイオテクノロジー企業、CRO、学術機関が存在するため、臨床試験画像処理の最大の市場です。この地域には、よく発達した医療インフラと大規模な患者プールもあります。
アジア太平洋地域 最も急速に成長している地域であると推定されています 臨床試験の画像処理 市場 予測期間2022-2029年
アジア太平洋地域は、臨床試験画像処理において最も急速に成長している市場です。この地域には人口が多く増加しており、医療に対する政府の資金提供が増加し、臨床試験に対する意識が高まっています。
臨床試験画像市場に関する詳細情報はこちら レポート、ここをクリック – https://www.databridgemarketresearch.com/reports/global-clinical-trial-imaging-market