Asia-Pacific Scleroderma Therapeutics Market Segmentation, By Drug Class (Immunosuppressants, Endothelin Receptor Antagonists, Phosphodiesterase 5 Inhibitors, Calcium Channel Blockers, Prostacyclin Analogues, and Others), Disease Type (Localized and Systemic), Route of Administration (Oral, Injectable, and Topical), End User (Hospitals, Specialty Clinics, Homecare, and Others), and Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies)- Industry Trends and Forecast to 2032
Scleroderma Therapeutics Market Size
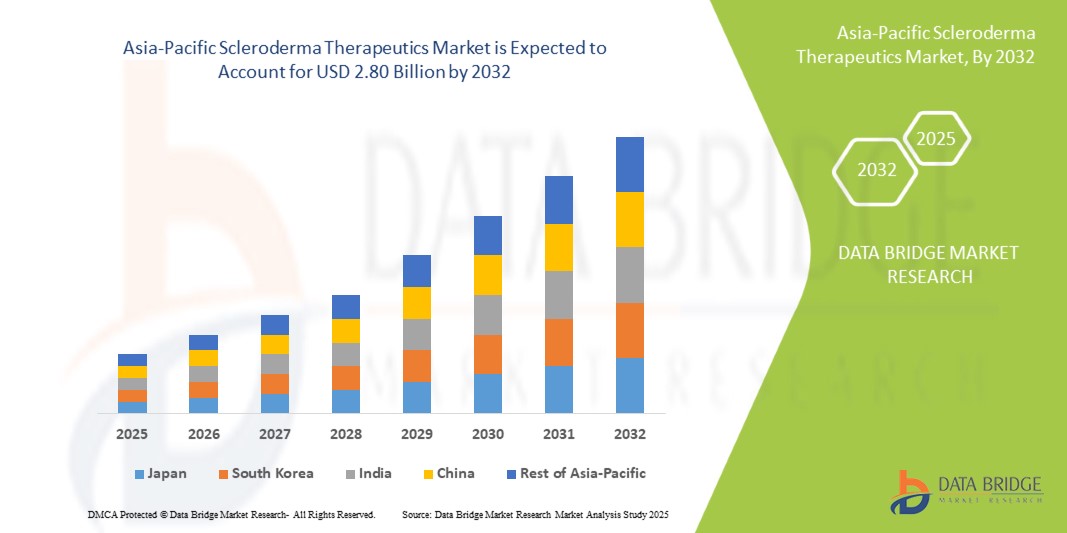
- The Asia-Pacific Scleroderma Therapeutics market size was valued atUSD 1.44 billion in 2024and is expected to reachUSD 2.80 billion by 2032, at aCAGR of 8.7%during the forecast period
- This growth is driven by increasing awareness about autoimmune disorders, rising incidence of systemic sclerosis, and improved access to targeted therapies across emerging Asian economies.
Scleroderma Therapeutics Market Analysis
- Scleroderma Therapeutics, also known as biotherapy or immunotherapy, uses living organisms or their components to treat diseases. These therapies have shown significant efficacy in managing cancer, autoimmune disorders, and genetic diseases due to their targeted action and reduced systemic side effects
- The demand for Scleroderma Therapeutics is significantly driven by rising incidences of cancer and autoimmune disorders, expanding approvals of biosimilars, and international investments in biotechnology innovation
- India is expected to dominate the Asia-Pacific Scleroderma Therapeutics market due to national rare disease programs, autoimmune disorder registries, and high treatment demand in systemic sclerosis.
- South Korea is expected to witness the highest CAGR in the Scleroderma Therapeutics market due to its growing biologics pipeline, clinical trial capacity, and government-backed healthcare innovation.
- Immunosuppressants segment is expected to hold the largest share of 42.5%, due to its widespread use in managing skin fibrosis and systemic complications. Expanding use of corticosteroids and methotrexate-based therapies is fueling demand in primary care settings.
Report Scope andScleroderma Therapeutics Market Segmentation
|
Attributes |
Scleroderma Therapeutics KeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Scleroderma Therapeutics Market Trends
“Integration of Real-World Evidence in Drug Evaluation”
The use of real-world evidence (RWE) is increasingly influencing clinical and regulatory decisions in the Asia-Pacific Scleroderma Therapeutics market. Real-world data, derived from electronic health records, observational studies, and patient registries, provides critical insights into long-term treatment outcomes and safety profiles outside the controlled environment of clinical trials. Regulatory agencies in countries likeJapanandSouth Koreaare actively promoting the integration of patient-reported outcomes and RWE into therapeutic assessments to accelerate drug approval timelines, especially for rare and autoimmune conditions. These initiatives are enabling more accurate benefit-risk evaluations and paving the way for conditional approvals of novel biologics for scleroderma treatment.
Scleroderma Therapeutics Market Dynamics
Driver
“Growing Prevalence of Systemic Sclerosis and Early Diagnosis”
- The Asia-Pacific region is witnessing a growing burden of systemic sclerosis, driven by environmental triggers, genetic predispositions, and increased clinical awareness. Government bodies and rheumatology associations acrossIndia, China, and Australiaare initiating early screening and referral programs to combat diagnostic delays. The rise in trained rheumatologists, along with improved access to autoimmune testing in tier 2 and 3 cities, has led to higher detection rates. Early diagnosis is crucial to initiating treatment before irreversible organ damage occurs.
- For instance,
In 2024,India’s Ministry of Health and Family Welfarelaunched a national autoimmune disorder registry to track conditions like systemic sclerosis. This initiative has enhanced the identification of early-stage patients, supported regional treatment planning, and promoted personalized medicine approaches across public healthcare facilities.
Opportunity
“Emergence of Biologics for Scleroderma Lung Complications”
- Lung-related complications such as interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) are among the leading causes of mortality in systemic sclerosis patients. The therapeutic landscape is rapidly evolving with the introduction of biologics that target fibrotic pathways, inflammatory cytokines, and vascular remodeling. Biologics likenintedanibandtocilizumabhave shown promise in slowing lung function decline and are being incorporated into treatment protocols across Asia-Pacific.
- For instance,
InNovember 2024,Boehringer Ingelheimannounced the expansion of its global ILD drug trial network to include clinical sites inIndia, South Korea, and Taiwan. This move aims to generate regional data to support regulatory approvals, improve representation of Asian patients in global trials, and enhance local treatment options.
Restraint/Challenge
“Limited Awareness and High Cost of Advanced Therapies”
- Despite advancements in therapeutics, awareness and accessibility remain major challenges, particularly in low- and middle-income countries across the Asia-Pacific region. Underdiagnosis of scleroderma in rural and semi-urban areas is common due to the lack of trained rheumatologists and limited availability of advanced diagnostic tools. Moreover, biologic therapies, while effective, are costly and often not covered under national insurance schemes, making them unaffordable for many patients.
- For instance,
In2023, a study published inThe Lancet Rheumatologyfocusing on scleroderma patients inIndiarevealed that the median time from symptom onset to diagnosis was18 months, primarily due to inadequate awareness and limited access to specialty care. Additionally, the high out-of-pocket expenses for biologics and immunomodulators have further hindered widespread adoption, necessitating policy reforms and the introduction of biosimilars to ensure affordability.
Scleroderma Therapeutics Market Scope
The market is segmented on the basis application, product type, technology, magnification type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Class |
|
|
By Disease Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
ByDistribution Channel
|
|
In 2025, the Immunosuppressants segment is projected to dominate the market with the largest share in drug class segment
In 2025, the Immunosuppressants segment is expected to hold the largest share of42.5%, due to its widespread use in managing skin fibrosis and systemic complications. Expanding use of corticosteroids and methotrexate-based therapies is fueling demand in primary care settings.
The Systemic Scleroderma segment is expected to account for the largest share during the forecast period in disease type segment
In 2025, the Systemic Scleroderma segment is anticipated to capture a63.4%share, driven by growing diagnosis of internal organ involvement and increased availability of systemic treatment options.
Scleroderma Therapeutics Market Regional Analysis
“India Holds the Largest Share in the Scleroderma Therapeutics Market”
- India is expected to dominate the Asia-Pacific Scleroderma Therapeutics market due to national rare disease programs, autoimmune disorder registries, and high treatment demand in systemic sclerosis.
- Additionally, India's large public health infrastructure and increasing affordability of generic immunosuppressants are boosting access to first-line therapies across tier 2 and tier 3 cities.
“South Korea is Projected to Register the HighestCAGR in the Scleroderma Therapeutics Market”
- South Korea is expected to witness the highest CAGR in the Scleroderma Therapeutics market due to its growing biologics pipeline, clinical trial capacity, and government-backed healthcare innovation.
- The country also benefits from strong academic-industry partnerships and digital health integration, which are accelerating early diagnosis and patient recruitment for rare disease research.
Scleroderma Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Boehringer Ingelheim International GmbH (Germany)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Sanofi S.A. (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bayer AG (Germany)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Gilead Sciences, Inc. (U.S.)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Actelion Pharmaceuticals Ltd (Switzerland)
Latest Developments in Asia-Pacific Scleroderma Therapeutics Market
- In February 2025, Mitsubishi Tanabe Pharma launched a new topical therapy for localized scleroderma in Japan, targeting pediatric patients.
- In January 2025, Roche expanded its Actemra (tocilizumab) scleroderma study to India and Indonesia as part of its APAC rare disease strategy.
- In October 2024, a multicenter trial evaluating sildenafil for digital ulcers in systemic sclerosis was launched across 5 Chinese hospitals.
- In March 2025, Pfizer Inc. partnered with the Japan Scleroderma Research Network (JSRN) to launch a post-marketing surveillance program evaluating long-term outcomes of immunosuppressants in systemic sclerosis patients, marking a strategic step towards real-world data collection and label expansion in the Japanese market.
- In April 2025, Roche initiated a multicenter Phase II clinical trial in Australia, Singapore, and South Korea for a novel antifibrotic monoclonal antibody targeting skin and lung fibrosis in systemic sclerosis, aiming to address unmet needs in severe scleroderma manifestations through precision biologics.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.