Global Point Of Care Diagnostics Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
43.94 Billion
USD
88.21 Billion
2024
2032
USD
43.94 Billion
USD
88.21 Billion
2024
2032
| 2025 –2032 | |
| USD 43.94 Billion | |
| USD 88.21 Billion | |
|
|
|
|
Global Point of Care Diagnostics Market Segmentation, By Platform (Lateral Flow Assays, Dipsticks, Microfluidics, Molecular Diagnostics, and Immunoassays), Prescription (Prescription-Based Testing, and OTC Testing), Product (Glucose, Cardio Metabolic, Infectious Disease, Influenza, HIV, Hepatitis C, Sexually Transmitted Disease (STD), Healthcare-Associated Infection (HAI), Respiratory Infection, Tropical Disease, Other Infectious Disease, Coagulation, PT/INR Activated Clotting Time (ACT/APTT), Pregnancy and Fertility, Tumor/Cancer Marker, Urinalysis, Cholesterol, Hematology, Drugs-of-Abuse, Fecal Occult, and Other POC Products), End-User (Professional Diagnostic Centers, Home Care, Research Laboratories, Other End Users)- Industry Trends and Forecast to 2032
Point of Care Diagnostics Market Size
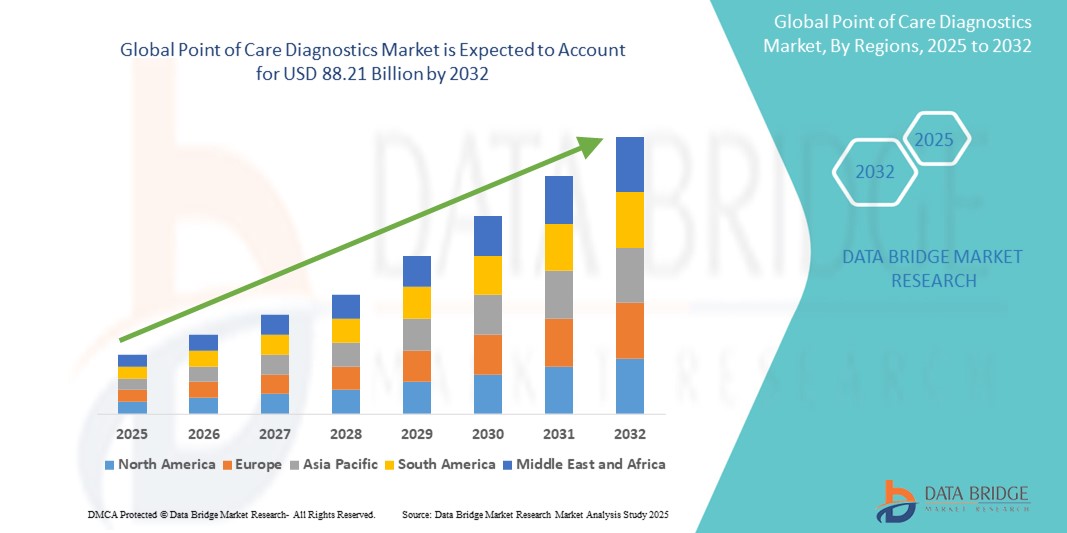
- The global point of care diagnostics market size was valued atUSD 43.94 billion in 2024and is expected to reachUSD 88.21 billion by 2032, at aCAGR of 9.1%during the forecast period
- This growth is driven by factors such as the increasing demand for rapid and accurate diagnostic tests, the rising prevalence of chronic diseases, and advancements in diagnostic technologies that improve patient outcomes and reduce healthcare costs
Point of Care Diagnostics Market Analysis
- Point of care diagnostics are medical testing devices that enable immediate diagnostic results at or near the site of patient care, enhancing rapid decision-making and treatment outcomes
- The demand for point of care diagnostics is significantly driven by the growing need for quick and accurate diagnostic solutions, increasing prevalence of chronic diseases, and advancements in diagnostic technologies
- North America is expected to dominate the point of care diagnostics market with a market share of 44.1 %, due to advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, and the presence of leading healthcare companies
- Asia-Pacific is expected to be the fastest growing region in the point of care diagnostics market with a market share of 23.5%, during the forecast period driven by rapid expansion in healthcare infrastructure, increasing awareness about early disease detection, and rising surgical and diagnostic volumes
- Infectious disease segment is expected to dominate the market with a market share of 25.9% due to its the rising global incidence of infectious diseases, including respiratory infections, sexually transmitted diseases, and emerging viral outbreaks
Report Scope and Point of Care Diagnostics Market Segmentation
|
Attributes |
Point of Care Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Point of Care Diagnostics Market Trends
“Advancements in Rapid Diagnostics & Integration of Artificial Intelligence”
- One prominent trend in the evolution of point of care diagnostics is the increasing integration of artificial intelligence (AI) and machine learning (ML) technologies to enhance diagnostic accuracy and speed
- These innovations enable real-time data analysis, allowing for quicker and more reliable diagnostic results, which is particularly valuable in emergency and remote care settings
- For instance, AI-powered diagnostic tools can assist in detecting a wide range of diseases, from infections to chronic conditions such as diabetes, by interpreting results from various testing devices with high precision and minimal human intervention
- These advancements are transforming healthcare delivery, improving patient outcomes, and driving the demand for next-generation point of care diagnostic devices that offer faster, more accurate results with enhanced user-friendly interfaces
Point of Care Diagnostics Market Dynamics
Driver
“Growing Demand Due to Rising Prevalence of Chronic Diseases”
- The increasing prevalence of chronic diseases such as diabetes, cardiovascular diseases, and infectious diseases is significantly contributing to the growing demand for point of care diagnostic devices
- As the global population faces a higher burden of chronic conditions, particularly with the aging population, the need for immediate and accessible diagnostic solutions becomes more urgent
- Chronic disease management requires continuous monitoring, and point of care diagnostics provide a fast and efficient way to monitor patients’ conditions in real-time, enhancing treatment outcomes
For instance,
- In a 2023 report by the World Health Organization, it was noted that approximately 422 million people worldwide suffer from diabetes, and the number is expected to rise, which will drive demand for glucose monitoring and other diagnostic tools
- As a result of the increasing prevalence of chronic diseases, there is a significant surge in the adoption of point of care diagnostics, leading to improved patient care and quicker medical interventions
Opportunity
“Advancing Healthcare with Artificial Intelligence Integration”
- AI-powered point of care diagnostic devices can enhance diagnostic accuracy, automate data analysis, and provide real-time insights, enabling healthcare providers to make more informed decisions and improve patient outcomes
- AI algorithms can analyze diagnostic test results, such as blood glucose levels, ECG readings, or imaging scans, and offer instant feedback, helping clinicians identify potential health risks or abnormalities early on
- Moreover, AI integration can streamline workflows, reduce diagnostic errors, and improve the overall efficiency of point of care testing, enabling healthcare professionals to deliver faster and more accurate care
For instance,
- In February 2025, a study published in Nature Medicine demonstrated that AI algorithms, specifically machine learning models, could accurately predict the onset of sepsis in patients by analyzing real-time data from point of care diagnostic devices. This AI-powered approach can significantly enhance early detection, leading to timely interventions and improved survival rates
- The integration of AI in point of care diagnostics presents a significant opportunity to improve diagnostic speed, reduce healthcare costs, and enhance the quality of patient care, particularly in remote and underserved areas where timely access to healthcare is critical
Restraint/Challenge
“High Equipment and Maintenance Costs Hindering Market Penetration”
- The high cost of point of care diagnostic devices and their maintenance poses a significant challenge to market growth, especially in low-resource settings and developing regions where healthcare budgets are limited
- Advanced point of care diagnostic devices, particularly those integrated with AI or advanced imaging capabilities, can be prohibitively expensive, often reaching several thousand dollars per unit
- This substantial financial barrier can limit the widespread adoption of point of care diagnostic devices, particularly in rural areas and developing countries, where cost-effective healthcare solutions are in high demand
For instance,
- In October 2024, according to a report by Frost & Sullivan, the high upfront costs and maintenance expenses of point of care diagnostic devices are cited as major obstacles to their adoption in emerging markets, potentially hindering access to vital healthcare services
- Consequently, such challenges can lead to limited access to advanced diagnostic technologies, exacerbating healthcare disparities and slowing the market's expansion in underdeveloped regions
Point of Care Diagnostics Market Scope
The market is segmented on the basis of platform, prescription, product and end user
|
Segmentation |
Sub-Segmentation |
|
By Platform |
|
|
By Prescription |
|
|
By Product |
|
|
By End User
|
|
In 2025, the infectious disease is projected to dominate the market with a largest share in product segment
The infectious disease segment is expected to dominate the point of care diagnostics market with the largest share of 25.9% in 2024 due to the rising global incidence of infectious diseases, including respiratory infections, sexually transmitted diseases, and emerging viral outbreaks. Point of care diagnostic tests offer rapid and accurate results, making them critical for timely diagnosis and treatment, especially in remote or resource-limited areas. The increasing focus on infectious disease management, coupled with the need for faster detection and prevention strategies, is driving the growth of this segment
The lateral flow assays is expected to account for the largest share during the forecast period in platform market
In 2025, the lateral flow assays segment is expected to dominate the market with the largest market share of 41% due to its simplicity, rapid turnaround time, and cost-effectiveness. These assays are widely used for infectious disease detection, pregnancy testing, and chronic disease monitoring. Their ease of use, minimal need for specialized equipment, and suitability for both clinical and home settings contribute to their widespread adoption globally.
Point of Care Diagnostics Market Regional Analysis
“North America Holds the Largest Share in the Point of Care Diagnostics Market”
- North America dominates thepoint of care diagnostics market with a market share of estimated 44.1%, driven, by advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, and the presence of leading healthcare companies
- U.S.holds a market share of 45.5%, due to the high demand for rapid diagnostics in emergency care, the increasing prevalence of chronic diseases such as diabetes and cardiovascular conditions, and continuous advancements in diagnostic technology
- The availability of robust reimbursement policies and ongoing investments in research and development by leading diagnostic manufacturers further strengthen the market
- In addition, the increasing number of testing procedures, including glucose monitoring, infectious disease testing, and pregnancy tests, is fueling market expansion across the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Point of Care Diagnostics Market”
- Asia-Pacific is expected to witness the highest growth rate in the Point of Care Diagnostics market with a market share of 23.5%, driven by rapid expansion in healthcare infrastructure, increasing awareness about early disease detection, and rising surgical and diagnostic volumes
- Countries such as China, India, and Japan are emerging as key markets due to the growing aging population and the rising burden of chronic and infectious diseases
- Japan, with its advanced medical technology and strong healthcare system, continues to lead in the adoption of innovative diagnostic tools, making it a crucial market for point of care diagnostics
- India is projected to register the highest CAGR in the point of care diagnostics market, driven by expanding healthcare infrastructure, rising healthcare needs due to a growing population, and increasing demand for affordable and efficient diagnostic solutions
Point of Care Diagnostics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Abbott(U.S.)
- Siemens Healthineers AG(Germany)
- QuidelOrtho Corporation.(U.S.)
- F. Hoffman-La Roche Ltd.(Switzerland)
- Danaher Corporation (U.S.)
- BD (U.S.)
- Chembio Diagnostics, Inc. (U.S.)
- EKF Diagnostics Holdings plc. (U.K.)
- Trinity Biotech (Ireland)
- Nova Biomedical (U.S.)
- PTS Diagnostics (U.S.)
- SEKISUI Diagnostics (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- bioMérieux S.A. (France)
- DiaSorin S.p.A (Italy)
- AccuBioTech Co., Ltd. (China)
- Meridian Bioscience, Inc. (U.S.)
- Biocartis (Europe)
- Terumo Corporation (Japan)
- Grifols, S.A. (Spain)
Latest Developments in Global Point of Care Diagnostics Market
- In February 2024, GE HealthCare launched the LOGIQ ultrasound portfolio, including the LOGIQ Totus, offering high-quality imaging and AI-powered diagnostic support to improve precision in point of care diagnostics
- In April 2024, Abbott's i-STAT TBI cartridge was approved for use with whole blood, enhancing rapid traumatic brain injury diagnostics at the point of care
- In January 2024, Rutgers University developed the CytoTracker Leukometer, a device that rapidly counts white blood cells from a single blood drop, aiding in swift sepsis detection and chemotherapy management
- In February 2022, Sense Biodetection entered into a strategic agreement with Una Health (Una) for the United Kingdom distribution of the Veros Covid-19 test
- In December 2021, FIND invested USD 21 million in Biomeme, Bioneer, Qlife, and SD Biosensor to accelerate the development, manufacturing, and launch of affordable point-of-care molecular diagnostic platforms that can detect multiple pathogens that cause diseases including COVID-19
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.