Mercado de tratamiento de enfermedades venosas de América del Norte, por tipo de producto (inyección de escleroterapia, dispositivos de ablación, productos de cierre venoso, stents venosos, medicamentos y otros), tipo de enfermedad (trombosis venosa profunda (TVP), insuficiencia venosa crónica (IVC), embolia pulmonar, tromboflebitis superficial, venas varicosas y otras), tipo de tratamiento (escleroterapia, terapia de ablación por radiofrecuencia, tratamiento con láser, flebectomía ambulatoria, ligadura y extracción de venas, angioplastia o colocación de stents, cirugías, terapia de compresión, medicación venoactiva, filtro de vena cava y otras terapias), usuario final (hospitales, clínicas, centros quirúrgicos ambulatorios y otros), canal de distribución (licitación directa, ventas minoristas y otros) - Tendencias de la industria y pronóstico hasta 2030.
Análisis y perspectivas del mercado de tratamiento de enfermedades venosas en América del Norte
La enfermedad venosa incluye coágulos de sangre en las piernas, brazos, cerebro, pulmones u órganos internos como riñones, bazo, hígado, trombosis venosa profunda, insuficiencia venosa crónica, venas varicosas y úlceras en las venas. El tratamiento para esta enfermedad incluye terapia con medicamentos, ablación láser endógena o ablación por radiofrecuencia (ARF), escleroterapia y cirugía.
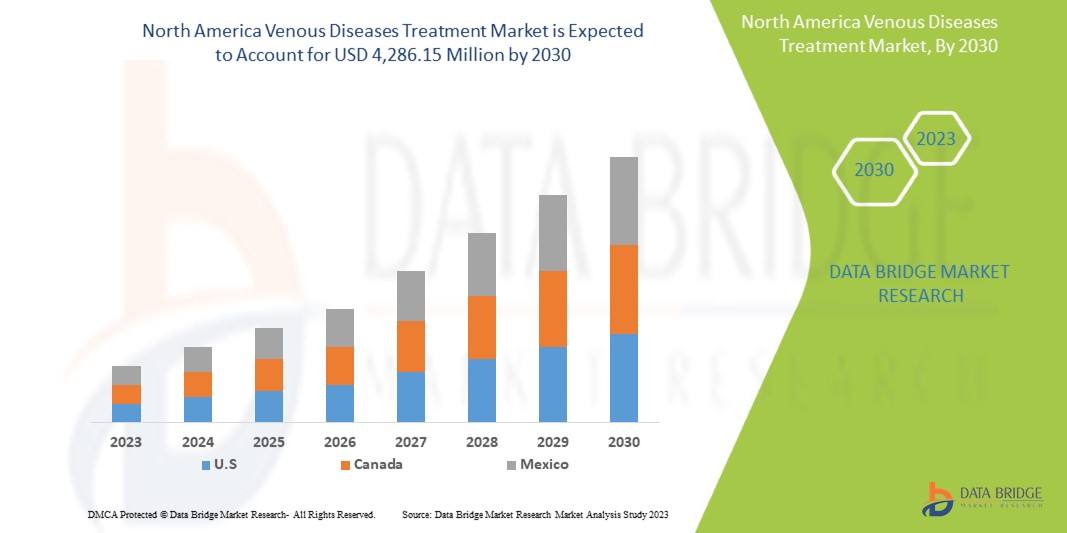
Data Bridge Market Research analiza que se espera que el mercado de tratamiento de enfermedades venosas de América del Norte alcance un valor de USD 4286,15 millones para 2030, con una CAGR del 7,4 % durante el período de pronóstico. Este informe de mercado también cubre en profundidad el análisis de precios, el análisis de patentes y los avances tecnológicos.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2020-1015) |
|
Unidades cuantitativas |
Ingresos en millones, precios en USD |
|
Segmentos cubiertos |
Por tipo de producto (inyección de escleroterapia, dispositivos de ablación, productos de cierre venoso, stents venosos, medicamentos y otros), tipo de enfermedad (trombosis venosa profunda [TVP], insuficiencia venosa crónica [IVC], embolia pulmonar, tromboflebitis superficial, venas varicosas y otras), tipo de tratamiento (escleroterapia, terapia de ablación por radiofrecuencia, tratamiento con láser, flebectomía ambulatoria, ligadura y extracción de venas, angioplastia o colocación de stents, cirugías, terapia de compresión, medicación venoactiva, filtro de vena cava y otras terapias), usuario final (hospitales, clínicas, centros quirúrgicos ambulatorios y otros), canal de distribución (licitación directa, ventas minoristas y otros) |
|
Países cubiertos |
Estados Unidos, Canadá y México. |
|
Actores del mercado cubiertos |
Abbott, Imricor, Baylis Medical Company, Inc., Theraclion, Sonablate, plusmedica.de, Boston Scientific Corporation, Olympus Corporation, Smith + Nephew, Cook, Scitech, Carl Zeiss Meditec AG, Teleflex built, Alma Lasers, BD, B.Braun SE, Medtronic, Stryker, Koninklijke Philips NV, Varian Medical Systems, Candela Corporation, Teromo corporation, Angiodynamics, optimed Medizinische Instrumente GmbH, Merit Medical Systems y Bolitec Laser, entre otros. |
Definición del mercado de tratamiento de enfermedades venosas en América del Norte
Los ataques cardíacos y los accidentes cerebrovasculares suelen ser eventos agudos y se deben principalmente a un bloqueo que impide el flujo sanguíneo al corazón o al cerebro. La causa más común es la acumulación de depósitos grasos en el revestimiento de los vasos sanguíneos que irrigan el corazón o el cerebro. Un accidente cerebrovascular puede ser causado por sangrado o coágulos de sangre en un vaso sanguíneo del cerebro.
Los avances tecnológicos en los stents vasculares, el aumento de la demanda de procedimientos mínimamente invasivos y el aumento de la población geriátrica están impulsando el mercado de América del Norte. Además, las empresas están ampliando su cartera de productos para ofrecer los mejores servicios para el tratamiento de enfermedades venosas. Los dispositivos médicos, como los dispositivos de ablación, se utilizan en procedimientos mínimamente invasivos para eliminar o extirpar tejidos corporales anormales con fines terapéuticos. Estos sistemas utilizan el calor generado por radiofrecuencia, energía, frío extremo o un láser para provocar pequeñas quemaduras. Se espera que el aumento de la adopción de tecnologías robóticas para la expansión de la aplicación de productos y la integración de tecnologías de vanguardia en dispositivos de ablación para mejorar la seguridad del paciente y la eficiencia del procedimiento impulsen el mercado.
Dinámica del mercado de tratamiento de enfermedades venosas en América del Norte
En esta sección se aborda la comprensión de los factores impulsores, las ventajas, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
Conductores
- Aumento de la incidencia de enfermedades venosas
Las enfermedades venosas son afecciones que dañan las venas del cuerpo. Las paredes dañadas de los vasos sanguíneos impiden que el sistema circulatorio funcione, lo que hace que la sangre se acumule y refluya (hacia atrás) cuando los músculos se relajan. Esto hace que se acumule una presión anormalmente alta en las venas. Esta acumulación hace que las venas se estrechen y se tuerzan, aumente la hinchazón, aumente la incompetencia de las válvulas, disminuya el flujo sanguíneo y se produzcan posibles coágulos sanguíneos. Por último, esta afección puede provocar diversas enfermedades conocidas como enfermedades venosas.
Debido a diversos factores de riesgo, como el envejecimiento, la obesidad, la presión arterial alta (hipertensión) o los antecedentes familiares de enfermedades venosas, el número de pacientes con enfermedades venosas está aumentando a nivel mundial y se está convirtiendo en un problema socioeconómico importante. Por lo tanto, el creciente número de pacientes con enfermedades venosas aumenta la demanda de tratamiento de enfermedades venosas, lo que actúa como un impulsor del mercado de tratamiento de enfermedades venosas en América del Norte.
- Los cambios rápidos en el estilo de vida conducen a la obesidad, lo que da lugar a enfermedades venosas.
Los cambios en el estilo de vida como el tabaquismo, una dieta poco saludable y la inactividad física conducen al desarrollo de enfermedades crónicas, especialmente enfermedades cardíacas, accidentes cerebrovasculares, diabetes, obesidad y síndrome metabólico que eventualmente pueden derivar en enfermedades venosas.
La actividad física es esencial y muchas enfermedades son consecuencia directa de un estilo de vida sedentario. El sedentarismo puede provocar aumento de peso, cansancio fácil y dolores y molestias inexplicables que derivan en enfermedades crónicas, como enfermedades cardiovasculares y venosas, que pueden ser moderadas, graves o incluso potencialmente mortales.
Según el artículo de ScienceDirect, la enfermedad venosa era clínicamente más grave en las extremidades obesas que en el grupo no obeso. Por lo tanto, debido al aumento en la adopción de un estilo de vida poco saludable, se ha producido un rápido aumento de la población obesa y de las enfermedades venosas. Por lo tanto, los rápidos cambios en el estilo de vida conducen a la obesidad, lo que da lugar a enfermedades venosas, lo que aumenta la demanda de tratamiento de enfermedades venosas y actúa como un impulsor del mercado de tratamiento de enfermedades venosas en América del Norte.
Restricción
- Falta de profesionales cualificados y certificados
La necesidad de profesionales cualificados y certificados es un gran obstáculo para el mercado de tratamiento de enfermedades venosas. La demanda de tratamientos para enfermedades venosas ha aumentado debido al aumento de casos de enfermedades venosas en Europa, pero la menor cantidad de profesionales cualificados presentes en los centros de atención sanitaria está obstaculizando el crecimiento del mercado.
Los profesionales sanitarios suelen pasar por alto la enfermedad venosa crónica (ECV) porque no comprenden la magnitud y el impacto del problema y no reconocen plenamente las diferentes manifestaciones de la enfermedad venosa primaria y secundaria. La importancia de las enfermedades cardiovasculares está relacionada con el número de pacientes y con los efectos socioeconómicos de sus manifestaciones más graves.
Oportunidad
- Concienciación sobre los trastornos venosos
Tanto los procedimientos médicos como los cambios en el estilo de vida pueden utilizarse para tratar los problemas de las venas. Las venas pueden beneficiarse de la medicación, el ejercicio y las medias de compresión, pero en ocasiones los problemas venosos requieren una terapia más exhaustiva para restablecer la salud y el funcionamiento de las venas. El curso del tratamiento está determinado por el tipo y la gravedad de la enfermedad venosa. El número de pacientes con trastornos vasculares ha aumentado drásticamente en las últimas décadas, siendo la diabetes el factor de riesgo más flagrante. A diferencia de otros problemas de salud, los trastornos vasculares y la cirugía vascular son desconocidos para aproximadamente el 80% de la población. Las actitudes y los comportamientos de las personas sobre enfermedades particulares pueden cambiar significativamente como resultado de una mayor conciencia de la salud.
Existen varios programas de concientización que llevan a cabo diversas sociedades, institutos gubernamentales y otros. Estas iniciativas que toman aumentarán la conciencia entre las personas sobre su salud y harán un diagnóstico temprano para una mejor cura y precaución. Por esta razón, se espera que la creciente conciencia sobre los trastornos venosos actúe como una oportunidad para aumentar la demanda del mercado de tratamiento de enfermedades venosas en América del Norte.
Desafío
- Alto costo asociado con el tratamiento de enfermedades venosas
Solo en Estados Unidos, más de 25 millones de personas padecen insuficiencia venosa crónica (IVC), y más de 6 millones padecen una enfermedad venosa avanzada. El sistema sanitario estadounidense está muy sobrecargado económicamente como resultado de la alta incidencia de la IVC y el aumento de los costes sanitarios. Numerosos problemas de salud generalizados son causados por una mala circulación venosa. El dolor de piernas, el edema y la pesadez son algunos de los primeros signos de la enfermedad venosa crónica (ECV), y pueden estar presentes durante todo el día o volverse más pronunciados por la noche. Los pacientes suelen buscar una terapia inicial para los síntomas que pueden presentarse con o sin varices, así como para la eliminación cosmética de las varices. Los dos principales factores de riesgo de la ECV son la edad avanzada y la masa corporal elevada.
Impacto posterior a la COVID-19 en el mercado de tratamiento de enfermedades venosas de América del Norte
La pandemia ha tenido efectos adversos tanto para los fabricantes como para los usuarios. Como los procedimientos para tratar las varices no son urgentes, el volumen de procedimientos disminuyó drásticamente. La reducción de las cirugías o procedimientos para el tratamiento de las varices debido a las restricciones de viaje también afectó las ventas de los fabricantes.
Los fabricantes están tomando diversas decisiones estratégicas para recuperarse tras la COVID-19. Los actores están llevando a cabo múltiples actividades de I+D y lanzamiento de productos y asociaciones estratégicas para mejorar la tecnología y los resultados de las pruebas involucradas en el mercado de sabores e ingredientes de alimentos para mascotas.
Acontecimientos recientes
- En julio de 2022, Smith+Nephew, la empresa de tecnología médica de América del Norte, lanzó la aplicación Clinical Support para ayudar a reducir la variación en la práctica del cuidado de heridas. La aplicación Clinical Support de WOUND COMPASS es una herramienta de apoyo digital integral para profesionales de la salud que ayuda a evaluar las heridas y a tomar decisiones para ayudar a reducir la variación en la práctica. Esto ha ayudado a la empresa a atraer a los clientes del sector sanitario y a ampliar su cartera de productos.
- En abril de 2022, Carl Zeiss Meditec anunció la adquisición de dos fabricantes de instrumentos quirúrgicos (Kogent Surgical, LLC y Katalyst Surgical, LLC) para fortalecer aún más su posicionamiento como proveedor de soluciones. Esto ha ayudado a la empresa a expandir su negocio.
Alcance del mercado de tratamiento de enfermedades venosas en América del Norte
El mercado de tratamiento de enfermedades venosas de América del Norte está segmentado por tipo de producto, tipo de enfermedad, tipo de tratamiento, usuario final y canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
MERCADO DE TRATAMIENTO DE ENFERMEDADES VENOSAS EN AMÉRICA DEL NORTE, POR TIPO DE PRODUCTO
- DISPOSITIVOS DE ABLACIÓN
- STENT VENOSOS
- PRODUCTOS PARA EL CIERRE VENOSO
- INYECCIÓN DE ESCLEROTERAPIA
- MEDICAMENTOS
- OTROS
Según el tipo de producto, el mercado de tratamiento de enfermedades venosas de América del Norte está segmentado en dispositivos de ablación, stents venosos, productos de cierre venoso, inyección de escleroterapia, medicamentos y otros.
MERCADO DE TRATAMIENTO DE ENFERMEDADES VENOSAS EN AMÉRICA DEL NORTE, POR TIPO DE ENFERMEDAD
- TROMBOSIS VENOSA PROFUNDA (TVP)
- INSUFICIENCIA VENOSA CRÓNICA (IVC)
- EMBOLIA PULMONAR
- TROMBOFLEBITIS SUPERFICIAL
- VARICES
- OTROS
Según el tipo de enfermedad, el mercado de tratamiento de enfermedades venosas de América del Norte se segmenta en trombosis venosa profunda (TVP), insuficiencia venosa crónica (IVC), embolia pulmonar, tromboflebitis superficial, venas varicosas y otras.
MERCADO DE TRATAMIENTO DE ENFERMEDADES VENOSAS EN AMÉRICA DEL NORTE, POR TIPO DE TRATAMIENTO
- TERAPIA DE COMPRESIÓN
- MEDICACIÓN VENOCTIVA
- CIRUGÍAS
- ESCLEROTERAPIA
- ANGIOPLASTIA O COLOCACIÓN DE STENTS
- LIGADURA Y EXTRACCIÓN DE VENAS
- FILTRO DE VENA CAVA
- FLEBECTOMÍA AMBULATORIA
- TERAPIA DE ABLACIÓN POR RADIOFRECUENCIA
- TRATAMIENTO LÁSER
- OTRAS TERAPIAS
Según el tipo de tratamiento, el mercado de tratamiento de enfermedades venosas de América del Norte se segmenta en terapia de compresión, medicación venoactiva, cirugías, escleroterapia, angioplastia o colocación de stents, ligadura y extracción de venas, filtro de vena cava, flebectomía ambulatoria, terapia de ablación por radiofrecuencia, tratamiento con láser y otras terapias.
MERCADO DE TRATAMIENTO DE ENFERMEDADES VENOSAS EN AMÉRICA DEL NORTE, POR USUARIO FINAL
- HOSPITALES
- CLÍNICAS
- CENTROS QUIRÚRGICOS AMBULATORIOS
- OTROS
Sobre la base del usuario final, el mercado de tratamiento de enfermedades venosas de América del Norte está segmentado en hospitales, clínicas, centros quirúrgicos ambulatorios y otros.
MERCADO DE TRATAMIENTOS PARA ENFERMEDADES VENOSAS EN AMÉRICA DEL NORTE, POR CANAL DE DISTRIBUCIÓN
- LICITACIÓN DIRECTA
- VENTAS AL POR MENOR
- OTROS
Sobre la base del canal de distribución, el mercado de tratamiento de enfermedades venosas de América del Norte está segmentado en licitación directa, ventas minoristas y otros.
Análisis y perspectivas regionales del mercado de tratamiento de enfermedades venosas en América del Norte
Se analiza el mercado de tratamiento de enfermedades venosas de América del Norte y se proporciona información sobre el tamaño del mercado, tipo de producto, tipo de enfermedad, tipo de tratamiento, usuario final y canal de distribución.
Los países cubiertos en este informe de mercado son Estados Unidos, Canadá y México.
Estados Unidos domina América del Norte debido a la presencia de actores clave en el mayor mercado de consumo con un alto PIB. Se espera que Estados Unidos crezca debido a su última tecnología avanzada e invenciones en el tratamiento de enfermedades venosas.
La sección de países del informe también proporciona factores de impacto individuales en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, se consideran la presencia y disponibilidad de marcas de América del Norte y los desafíos que enfrentan debido a la competencia grande o escasa de las marcas locales y nacionales, y el impacto de los canales de venta al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de tratamiento de enfermedades venosas en América del Norte
El panorama competitivo del mercado de tratamiento de enfermedades venosas de América del Norte proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en I+D, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y amplitud de los productos, el dominio de las aplicaciones y la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa en el mercado de tratamiento de enfermedades venosas de América del Norte.
Algunos de los principales actores que operan en el mercado de tratamiento de enfermedades venosas de América del Norte son Abbott, Imricor, Baylis Medical Company, Inc., Theraclion, Sonablate, plusmedica.de, Boston Scientific Corporation, Olympus Corporation, Smith + Nephew, Cook, Scitech, Carl Zeiss Meditec AG, Teleflex Incorporated, Alma Lasers, BD, B.Braun SE, Medtronic, Stryker, Koninklijke Philips NV, Varian Medical Systems, Candela Corporation, Teromo Corporation, Angiodynamics, optimed Medizinische Instrumente GmbH, Merit Medical Systems, Bolitec Laser, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 INSTRUMENT BASED LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING INCIDENCES OF VENOUS DISEASES
8.1.2 RAPID CHANGES IN LIFESTYLE LEAD TO OBESITY RESULTING IN VENOUS DISEASES
8.1.3 INCREASE IN THE GERIATRIC POPULATION
8.1.4 TECHNOLOGICALLY ADVANCEMENT IN THE TREATMENT OF VENOUS DISEASES
8.2 RESTRAINTS
8.2.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
8.2.2 INADEQUATE REIMBURSEMENT COVERAGE
8.3 OPPORTUNITIES
8.3.1 RISING AWARENESS TOWARDS VENOUS DISORDERS
8.3.2 NEED FOR PROPER DIAGNOSIS AND TREATMENT OF VENOUS DISEASES
8.3.3 GROWING PREFERENCE FOR MINIMALLY INVASIVE PROCEDURES
8.3.4 OCCUPATIONAL LIFESTYLE INCREASES THE NEED FOR VENOUS DISEASE TREATMENT
8.4 CHALLENGES
8.4.1 HIGH COST ASSOCIATED WITH VENOUS DISEASE TREATMENT
8.4.2 SIDE EFFECTS AND RISK ASSOCIATED WITH DIFFERENT TREATMENT MODES
9 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 SCLEROTHERAPY INJECTION
9.2.1 SCLEROTHERAPY, BY TYPE
9.2.1.1 INTRAVENOUS
9.2.1.2 INTRADERMAL
9.2.1.3 SUBCUTANEOUS
9.2.2 SUBCUTANEOUS, BY APPLICATION
9.2.2.1 MALFORMED LYMPHED VESSELS
9.2.2.2 HEMORRHOIDS
9.2.2.3 HYDROCELES
9.2.2.4 OTHERS
9.3 ABLATION DEVICES
9.3.1 THERMAL ABLATION
9.3.1.1 RADIOFREQUENCY
9.3.1.1.1 CATHETER MANIPULATION SYSTEMS
9.3.1.1.2 TEMPERATURE CONTROLLED
9.3.1.1.3 FLUID COOLED
9.3.1.2 LIGHT
9.3.1.2.1 EXCIMER LASERS
9.3.1.2.2 COLD LASERS
9.3.1.3 ULTRASOUND
9.3.1.3.1 HIGH INTENSITY FOCUSED ULTRASOUND (HIFU)
9.3.1.3.2 SHOCK WAVE LITHOTRIPSY
9.3.1.3.3 MAGNETIC RESONANCE IMAGING GUIDED FOCUSED ULTRASOUND (MRI-FUS)
9.3.1.3.4 ULTRASONIC SURGICAL SYSTEMS
9.3.1.4 RADIATION
9.3.1.4.1 STEREOTACTIC BODY RADIATION THERAPY
9.3.1.4.2 INTENSITY-MODULATED RADIATION THERAPY
9.3.1.4.3 STEREOTACTIC RADIOTHERAPY & RADIOSURGERY
9.3.1.4.4 IMAGE GUIDED RADIATION THERAPY
9.3.1.4.5 INTRAVASCULAR BRACHYTHERAPY
9.3.1.4.6 PROTON BEAM THERAPY
9.3.1.5 ELECTRICAL
9.3.1.5.1 ELECTRICAL ABLATORS
9.3.1.5.2 ELECTRONIC BRACHYTHERAPY
9.3.1.6 MICROWAVE
9.3.1.7 HYDROTHERMAL
9.3.2 NON-THERMAL ABLATION
9.3.2.1 CRYOABLATION
9.3.2.1.1 EPIDERMAL AND SUBCUTANEOUS CRYOABLATION DEVICES
9.3.2.1.2 CRYOGEN SPRAY PROBE
9.3.2.1.3 TISSUE CONTACT PROBE
9.3.2.2 HYDROMECHANICAL ABLATION
9.4 VENOUS CLOSURE PRODUCTS
9.4.1 VENOUS CLOSURE PRODUCTS, BY PROCEDURE
9.4.1.1 INTERVENTIONAL CARDIOLOGY
9.4.1.2 INTERVENTIONAL RADIOLOGY
9.4.2 VENOUS CLOSURE PRODUCTS, BY TECHNOLOGY
9.4.2.1 FEMORAL ACCESS TECHNIQUE
9.4.2.2 RADIAL ACCESS TECHNIQUE
9.5 VENOUS STENTS
9.5.1 VENOUS STENTS, BY TECHNOLOGY
9.5.1.1 WALLSTENT TECHNOLOGY
9.5.1.2 ILIAC VEIN STENT TECHNOLOGY
9.5.2 VENOUS STENTS, BY APPLICATION
9.5.2.1 LEG
9.5.2.2 CHEST
9.5.2.3 ABDOMEN
9.5.2.4 OTHERS
9.6 MEDICATION
9.7 OTHERS
10 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 VARICOSE VEINS
10.3 DEEP VEIN THROMBOSIS (DVT)
10.4 PULMONARY EMBOLISM
10.5 CHRONIC VENOUS INSUFFICIENCY (CVI)
10.6 SUPERFICIAL THROMBOPHLEBITIS
10.7 OTHERS
11 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE
11.1 OVERVIEW
11.2 SCLEROTHERAPY
11.3 RADIOFREQUENCY ABLATION THERAPY
11.4 LASER TREATMENT
11.5 AMBULATORY PHLEBECTOMY
11.6 VEIN LIGATION AND STRIPPING
11.7 ANGIOPLASTY OR STENTING
11.8 SURGERIES
11.9 COMPRESSION THERAPY
11.1 VEINACTIVE MEDICATION
11.11 VENA CAVA FILTER
11.12 OTHER THERAPIES
12 NORTH AMERICA VENOUS TREATMENT DISEASES MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
13.4 OTHERS
14 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 MEDTRONIC
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.2 BOSTON SCIENTIFIC CORPORATION
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 VARIAN MEDICAL SYSTEMS, INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 COMPANY SHARE ANALYSIS
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENT
17.4 STRYKER
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 KONINKLIJKE PHILIPS N.V.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 ABBOTT
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 ALMA LASERS
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 ANGIODYNAMICS
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENT
17.9 B.BRAUN MELSUNGEN AG
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 BAYLIS MEDICAL COMPANY, INC
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 BD
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 CANDELA MEDICAL
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.13 CARL ZEISS MEDITEC AG
17.13.1 COMPANYSNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 COOK
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENTS
17.15 IMRICOR
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 OLYMPUS CORPORATION
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOILIO
17.16.4 RECENT DEVELOPMENT
17.17 OPTIMED MEDIZINISCHE INSTRUMENTE GMBH
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENTS
17.18 PLUSMEDICA.DE
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 SCITECH
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENTS
17.2 SMITH + NEPHEW
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.21 SONABLATE
17.21.1 COMPANY SNAPSHOT
17.21.2 PRODUCT PORTFOLIO
17.21.3 RECENT DEVELOPMENT
17.22 THERACLION
17.22.1 COMPANY SNAPSHOT
17.22.2 REVENUE ANALYSIS
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENT
17.23 TELEFLEX INCORPORATED
17.23.1 COMPANY SNAPSHOT
17.23.2 REVENUE ANALYSIS
17.23.3 PRODUCT PORTFOLIO
17.23.4 RECENT DEVELOPMENTS
17.24 TERUMO CORPORATION
17.24.1 COMPANY SNAPSHOT
17.24.2 REVENUE ANALYSIS
17.24.3 PRODUCT PORTFOLIO
17.24.4 RECENT DEVELOPMENTS
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tablas
TABLE 1 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA ILIAC VEIN STENT TECHNOLOGY IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA MEDICATION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA VARICOSE VEINS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA DEEP VEIN THROMBOSIS (DVT) IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA PULMONARY EMBOLISM IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA CHRONIC VENOUS INSUFFICIENCY (CVI) IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA SUPERFICIAL THROMBOPHLEBITIS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA SCELEROTHERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA RADIOFREQUENCY ABLATION THERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA LASER TREATMENT IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA AMBULATORY PHELEBECTOMY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA VEIN LIGATION AND STRIPPING IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA ANGIOPLASTY OR STENTING IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA SURGERIES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA COMPRESSION THERAPY IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA VEINACTIVE MEDICATION IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA VENA CAVA FILTER IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA OTHER THERAPIES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA HOSPITALS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CLINICS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIRECT TENDER IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA RETAIL SALES IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA OTHERS IN VENOUS DISEASES TREATMENT MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 72 U.S. VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 U.S. SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 U.S. SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 75 U.S. ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 U.S. THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 77 U.S. RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 78 U.S. LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 U.S. ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.S. RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.S. ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.S. NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.S. CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.S. VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 85 U.S. VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 86 U.S. VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 87 U.S. VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 88 U.S. VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 89 U.S. VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 90 U.S. VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 U.S. VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 92 CANADA VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 93 CANADA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 CANADA SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 95 CANADA ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CANADA RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 CANADA LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 CANADA ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 CANADA RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 CANADA ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 CANADA NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 CANADA CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 CANADA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 104 CANADA VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 105 CANADA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 106 CANADA VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 107 CANADA VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 108 CANADA VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 109 CANADA VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 110 CANADA VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 111 MEXICO VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 112 MEXICO SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 113 MEXICO SCLEROTHERAPY INJECTION IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 114 MEXICO ABLATION DEVICES IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 MEXICO THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 MEXICO RADIOFREQUENCY IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 MEXICO LIGHT IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 MEXICO ULTRASOUND IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 MEXICO RADIATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MEXICO ELECTRICAL IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 MEXICO NON-THERMAL ABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 MEXICO CRYOABLATION IN VENOUS DISEASES TREATMENT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 123 MEXICO VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY PROCEDURE, 2021-2030 (USD MILLION)
TABLE 124 MEXICO VENOUS CLOSURE PRODUCTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 125 MEXICO VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 126 MEXICO VENOUS STENTS IN VENOUS DISEASES TREATMENT MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 127 MEXICO VENOUS DISEASES TREATMENT MARKET, BY DISEASE TYPE, 2021-2030 (USD MILLION)
TABLE 128 MEXICO VENOUS DISEASES TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD MILLION)
TABLE 129 MEXICO VENOUS DISEASES TREATMENT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 MEXICO VENOUS DISEASES TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS ABOUT VENOUS DISEASES TREATMENTS AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET FROM 2023 TO 2030
FIGURE 12 THE ABLATION DEVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE NORTH AMERICA VENOUS DISEASES TREATMENT MARKET
FIGURE 14 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, 2022
FIGURE 19 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, 2022
FIGURE 23 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, 2022
FIGURE 27 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: SNAPSHOT (2022)
FIGURE 35 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2022)
FIGURE 36 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 NORTH AMERICA VENOUS DISEASES TREATMENT MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.