Mercado de diagnóstico de cáncer de páncreas en América del Norte, por tipo de prueba (prueba de imagen, biopsia, análisis de sangre, prueba genómica y otras), estadio del cáncer (estadio 0, estadio I, estadio II, estadio III y estadio IV), tipo de tumor (tumores exocrinos y tumores neuroendocrinos), producto (productos basados en instrumentos, productos basados en plataformas, kits y reactivos y otros consumibles), tecnología (hibridación fluorescente in situ, secuenciación de próxima generación, fluoroinmunoensayo, hibridación genómica comparativa, inmunohistoquímica y otras), aplicación (detección, diagnóstico y predicción, pronóstico e investigación), usuario final (hospitales, centros de diagnóstico, centros de investigación del cáncer, institutos académicos, centros quirúrgicos ambulatorios y otros), canal de distribución (licitación directa, ventas minoristas y otros), tendencias de la industria y pronóstico hasta 2030.
Análisis y perspectivas del mercado de diagnóstico de cáncer de páncreas en América del Norte
La creciente prevalencia del cáncer de páncreas, así como la creciente necesidad de productos de diagnóstico para estas enfermedades, han aumentado la demanda del mercado. El avance de la tecnología para facilitar el suministro de productos y las instalaciones de fabricación rápidas también contribuyen al crecimiento del mercado. Los principales actores del mercado se centran en gran medida en los lanzamientos y las aprobaciones de productos durante este período crucial. Además, el gobierno y los organismos reguladores están apoyando a los actores del mercado mediante la aprobación de productos debido al aumento de la aparición de nuevos productos.
Se espera que el mercado de diagnóstico de cáncer de páncreas de América del Norte crezca en el año de pronóstico debido al aumento de los actores del mercado y la disponibilidad de servicios avanzados. Junto con esto, los fabricantes participan en la actividad de I + D para lanzar nuevos servicios en el mercado. Se espera que la creciente investigación en el campo del diagnóstico y desarrollo de la leucemia impulse aún más el crecimiento del mercado. Sin embargo, se espera que las dificultades en las técnicas de detección de leucemia obstaculicen el crecimiento del mercado de diagnóstico de cáncer de páncreas de América del Norte en el período de pronóstico. Se espera que el aumento del gasto sanitario en el diagnóstico y tratamiento del cáncer brinde oportunidades al mercado para mejorar el tratamiento. Se espera que la mejora en la conciencia sobre los controles sanitarios regulares, los próximos centros de diagnóstico y los avances en los métodos de diagnóstico para el cáncer de páncreas y los desarrollos tecnológicos impulsen el crecimiento del mercado. Sin embargo, se espera que el alto costo de las pruebas y las estrictas regulaciones y estándares para la aprobación y comercialización de productos e instrumentos de diagnóstico del cáncer desafíen el crecimiento del mercado.
La creciente población geriátrica, las iniciativas estratégicas de los actores del mercado y del gobierno y el aumento del gasto en atención médica brindan oportunidades de mercado para mejorar el tratamiento. Sin embargo, la falta de profesionales capacitados y marcos regulatorios estrictos son desafíos clave para el crecimiento del mercado. Sin embargo, se espera que el alto costo de los dispositivos y tratamientos frene el crecimiento del mercado de diagnóstico de cáncer de páncreas en América del Norte.
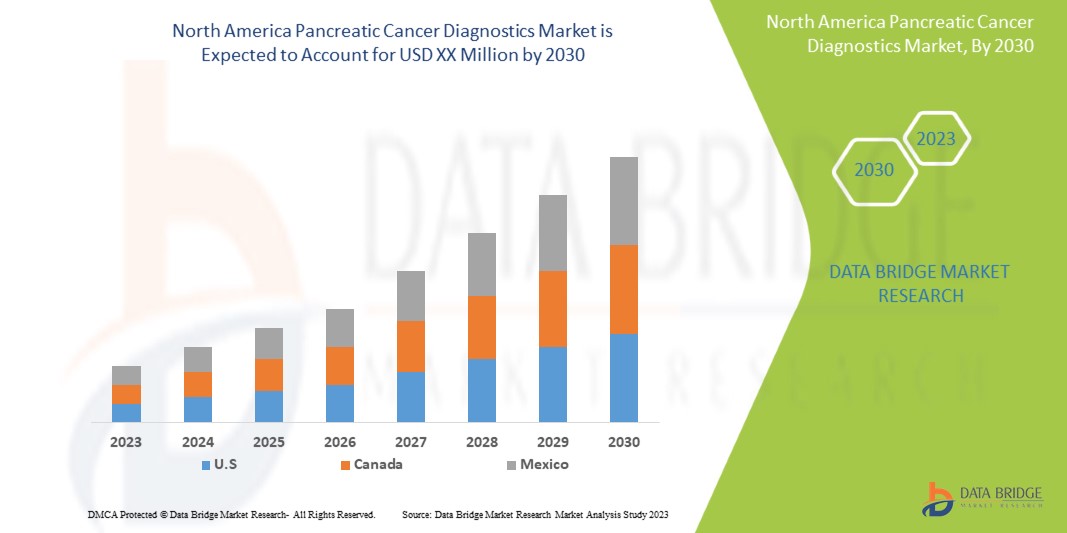
El mercado de diagnóstico de cáncer de páncreas de América del Norte es favorable y tiene como objetivo reducir la enfermedad, mejorando así la recuperación y el rendimiento de las personas. Data Bridge Market Research analiza que el mercado de diagnóstico de cáncer de páncreas de América del Norte crecerá a una CAGR del 7,7 % durante el período de pronóstico de 2023 a 2030.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2020-2016) |
|
Unidades cuantitativas |
Ingresos en millones de USD, precios en USD |
|
Segmentos cubiertos |
Por tipo de prueba (prueba de imagen, biopsia, análisis de sangre, prueba genómica y otras), estadio del cáncer (estadio 0, estadio I, estadio II, estadio III y estadio IV), tipo de tumor (tumores exocrinos y tumores neuroendocrinos ), producto (productos basados en instrumentos, productos basados en plataformas, kits y reactivos y otros consumibles), tecnología (hibridación fluorescente in situ, secuenciación de próxima generación, fluoroinmunoensayo, hibridación genómica comparativa, inmunohistoquímica y otras), aplicación (detección, diagnóstico y predicción, pronóstico e investigación), usuario final (hospitales, centros de diagnóstico, centros de investigación del cáncer, institutos académicos, centros de cirugía ambulatoria y otros), canal de distribución (licitación directa, ventas minoristas y otros). |
|
País cubierto |
Estados Unidos, Canadá, México. |
|
Actores del mercado cubiertos |
Siemens Healthcare Private Limited, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., entre otros. |
Definición de mercado
El cáncer de páncreas es mortal y su diagnóstico también plantea problemas de seguridad, ya que no es rentable. Uno de los trastornos médicos más costosos de tratar en Norteamérica es el cáncer. Los pacientes con cáncer pueden ser hospitalizados y recibir una variedad de terapias para el tumor, como cirugía, radioterapia y terapia sistémica. Las primas de seguro médico para pacientes con cáncer son ahora más caras que en el pasado. Además, sus copagos, deducibles y coaseguros están aumentando. El diagnóstico del cáncer de páncreas incluye ecografías, procedimientos de biopsia y análisis de sangre. El cáncer de páncreas es una de las principales causas de muerte en todo el mundo y su prevalencia ha aumentado a un ritmo alarmante.
Dinámica del mercado de diagnóstico de cáncer de páncreas en América del Norte
En esta sección se aborda la comprensión de los factores impulsores, las oportunidades, las limitaciones y los desafíos del mercado. Todos ellos se analizan en detalle a continuación:
Conductores
- Aumenta la prevalencia del cáncer de páncreas
Este tipo de cáncer puede afectar a personas de todas las edades. El cáncer de páncreas puede ser difícil de diagnosticar porque, a pesar de su amplia gama de signos y síntomas, no son específicos y pueden estar relacionados con otras afecciones médicas más extendidas. El cáncer de páncreas es el octavo cáncer más común en mujeres y el décimo cáncer más común en hombres. Las tasas de incidencia del cáncer de páncreas han aumentado alrededor de un 1% cada año. Se presenta con menos frecuencia. Es ligeramente más común entre las mujeres que entre los hombres, sin embargo, el riesgo promedio de por vida de contraer cáncer de páncreas en ambos sexos es de alrededor de ½ del 1% en promedio. Estas afecciones incluyen: dolor abdominal, pérdida de apetito o pérdida de peso involuntaria, coloración amarillenta de la piel y el blanco de los ojos (ictericia), heces de color claro, orina de color oscuro y picazón en la piel. Es el octavo tipo de cáncer más común diagnosticado en adultos y niños, pero la mayoría de los casos se presentan en adultos. Aunque se puede diagnosticar a cualquier edad, es poco común antes de los 45 años. La edad promedio de diagnóstico es los 68 años.
Debido a diversos factores de riesgo, la incidencia del cáncer de páncreas ha aumentado en América del Norte y se ha convertido en un problema socioeconómico importante. Se espera que esto actúe como un factor impulsor del mercado de diagnóstico del cáncer de páncreas en América del Norte.
- Nuevos avances tecnológicos en el diagnóstico pancreático
El cáncer de páncreas rara vez se detecta en sus primeras etapas, cuando es más curable, ya que a menudo no causa síntomas hasta que se ha propagado a otros órganos. Los especialistas deben diagnosticar manualmente las células cancerosas y no cancerosas examinando imágenes de células bajo un microscopio y proporcionando etiquetas mediante anotaciones. Sin embargo, este examen microscópico manual requiere mucho tiempo y puede dar un diagnóstico incorrecto. El riesgo de prescribir medicamentos incorrectos se redujo mediante el uso de software informático. La creación de un sistema de clasificación automático y confiable se volvió vital para detener los efectos devastadores de la enfermedad pancreática. Las técnicas de segmentación múltiple constituyeron la base de los algoritmos de clasificación del cáncer de páncreas existentes.
Oportunidad
- Aumento del gasto sanitario para el diagnóstico y tratamiento del cáncer
En todo el mundo, las actividades de investigación y desarrollo están aumentando debido al gasto público en salud con resultados económicos. Mientras que la industria de la salud ocupa el segundo lugar entre todas las industrias en lo que respecta a la cantidad gastada en atención médica. El aumento del gasto en atención médica puede dar como resultado una mejor provisión de oportunidades de investigación y desarrollo. Se prevé que aumente la demanda de diagnósticos de cáncer de páncreas. El aumento del gasto en atención médica para el tratamiento del cáncer de páncreas también ayuda al paciente a recibir diagnósticos y tratamientos avanzados sin complicaciones para una recuperación rápida. El gasto en atención médica se compone de la combinación de pagos de bolsillo (personas que pagan por su atención), gasto gubernamental y fuentes. También incluye seguros médicos y actividades de organizaciones no gubernamentales. Este aumento del gasto en atención médica para el tratamiento del cáncer es una oportunidad para la demanda del mercado.
Restricción/Desafío
- Diagnóstico tardío y mal pronóstico del cáncer de páncreas
El diagnóstico tardío de la enfermedad se debe a que los tumores de cáncer de páncreas, que aumentan, no responden tan bien a las terapias contra el cáncer que se utilizan habitualmente como otros tipos de cáncer menos letales, pero existen opciones de tratamiento, como la cirugía, la quimioterapia y la radiación. Existen diferentes tipos de cáncer de páncreas. La mayoría de los cánceres de páncreas son de tipo exocrino, es decir, que se originan en las células que producen los jugos digestivos pancreáticos. Alrededor del 30 por ciento de los pacientes son fumadores y el 5 por ciento tiene antecedentes de pancreatitis, una inflamación del páncreas que puede ser causada por cálculos o por el consumo excesivo de alcohol.
Impacto posterior al COVID-19 en el mercado de diagnóstico de cáncer de páncreas en América del Norte
La COVID-19 ha afectado negativamente al crecimiento del mercado, ya que los pacientes que padecen cáncer de páncreas pospusieron su cirugía debido al rápido aumento de casos de COVID-19 en todas las geografías. Además, las personas que padecen cáncer de páncreas corren el riesgo de enfermarse gravemente. El miedo a la infección por coronavirus afectó el crecimiento del mercado de diagnóstico del cáncer de páncreas en medio de la pandemia.
Acontecimientos recientes
- En diciembre de 2022, FUJIFILM Holdings America Corporation anunció que la empresa ha firmado un acuerdo de compra de activos con Inspirata, Inc. para adquirir el negocio de patología digital y ampliar su sólida oferta de imágenes empresariales. Esto permite la integración de imágenes y datos patológicos en el sistema de registros médicos electrónicos de una organización sanitaria para agilizar la prestación de atención a los pacientes oncológicos.
- En agosto de 2020, Siemens Healthcare GmbH anunció que había firmado un acuerdo con Varian Medical Systems, Inc. Con esta adquisición, Siemens Healthcare ha ayudado a desarrollar soluciones avanzadas para tratar el cáncer y fortalecer su posición en la industria de la salud.
Alcance del mercado de diagnóstico de cáncer de páncreas en América del Norte
El mercado de diagnóstico de cáncer de páncreas de América del Norte se clasifica en ocho segmentos notables según el tipo de prueba, las etapas del cáncer, el tipo de tumor, el producto, la aplicación, la tecnología, el usuario final y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
Tipo de prueba
- Prueba de imagen
- Biopsia
- Análisis de sangre
- Prueba genómica
- Otros
Según el tipo de prueba, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en pruebas de imagen, biopsia, análisis de sangre, prueba genómica y otras.
Estadio del cáncer
- Etapa 0
- Etapa I
- Estadio II
- Estadio III
- Estadio IV
Sobre la base de la etapa del cáncer, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en etapa 0, etapa I, etapa II, etapa III y etapa IV.
Tipo de tumor
- Tumores exocrinos
- Tumores neuroendocrinos
Según el tipo de tumor, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en tumores exocrinos y tumores neuroendocrinos.
Producto
- Productos basados en instrumentos
- Productos basados en plataformas
- Kits y reactivos
- Otros consumibles
Sobre la base del producto, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en productos basados en instrumentos, productos basados en plataformas, kits y reactivos y otros consumibles.
Solicitud
- Cribado
- Diagnóstico y predictivo
- Pronóstico
- Investigación
Sobre la base de la aplicación, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en detección, diagnóstico y predicción, pronóstico e investigación.
Tecnología
- Hibridación fluorescente in situ
- Secuenciación de próxima generación
- Fluoroinmunoensayo
- Hibridación genómica comparativa
- Inmunohistoquímica
- Otros
Sobre la base de la tecnología, el mercado de diagnóstico de cáncer de páncreas en América del Norte está segmentado en hibridación fluorescente in situ, secuenciación de próxima generación, fluoroinmunoensayo, hibridación genómica comparativa, inmunohistoquímica y otros.
Usuario final
- Hospitales
- Centros de diagnóstico
- Centros de investigación del cáncer
- Institutos académicos
- Centros de cirugía ambulatoria
- Otros
Sobre la base del usuario final, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en hospitales, centros de diagnóstico, centros de investigación del cáncer, institutos académicos, centros quirúrgicos ambulatorios y otros.
Canal de distribución
- Licitación directa
- Ventas al por menor
- Otros
Sobre la base del canal de distribución, el mercado de diagnóstico de cáncer de páncreas de América del Norte está segmentado en licitación directa, ventas minoristas y otros.
Análisis y perspectivas del mercado de diagnóstico de cáncer de páncreas en América del Norte
Se analiza el mercado de diagnóstico de cáncer de páncreas de América del Norte y se proporcionan información y tendencias sobre el tamaño del mercado por país, tipo de prueba, estadios del cáncer, tipo de tumor, producto, aplicación, tecnología, usuario final y canal de distribución como se menciona anteriormente.
- Se espera que en 2023, el mercado de diagnóstico de cáncer de páncreas en EE. UU. crezca debido al aumento de la prevalencia e incidencia del cáncer de páncreas y al aumento de la conciencia sobre el diagnóstico de cáncer de páncreas. Estos son los factores clave que se espera que impulsen el crecimiento del mercado en el país.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en la regulación del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como el análisis de la cadena de valor aguas abajo y aguas arriba, las tendencias técnicas, el análisis de las cinco fuerzas de Porter y los estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, y el impacto de los aranceles nacionales y las rutas comerciales se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de diagnóstico del cáncer de páncreas en América del Norte
El panorama competitivo del mercado de diagnóstico de cáncer de páncreas en América del Norte proporciona detalles por competidores. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y la variedad de productos y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa en el mercado de diagnóstico de cáncer de páncreas en América del Norte.
Algunos de los principales actores que operan en el mercado son Siemens Healthcare Private Limited, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROW IN PREVALENCE OF PANCREATIC CANCER
8.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN PANCREATIC DIAGNOSTICS
8.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
8.1.4 INCREASE IN AWARENESS REGARDING PANCREATIC CANCER
8.2 RESTRAINTS
8.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF PANCREATIC CANCER DIAGNOSTIC PRODUCTS
8.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF PANCREATIC CANCER
8.3 OPPORTUNITIES
8.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR PANCREATIC CANCER
8.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
8.3.3 GOVERNMENT INITIATIVES TOWARD PANCREATIC CANCER DIAGNOSTICS
8.4 CHALLENGES
8.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
8.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MAGNETIC RESONANCE IMAGING (MRI)
9.2.2.1 MR CHOLANGIOPANCREATOGRAPHY
9.2.2.2 MR ANGIOGRAPHY (MRA)
9.2.3 ULTRASOUND
9.2.3.1 ABDOMINAL ULTRASOUND
9.2.3.2 ENDOSCOPIC ULTRASOUND (EUS)
9.2.4 CHOLANGIOPANCREATOGRAPHY
9.2.4.1 MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY (MRCP)
9.2.4.2 ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP)
9.2.4.3 PRECUTANEOUS TRANSHEPTIC CHOLANGIOPANCREATOGRAPHY (PTC)
9.2.5 POSITRON EMISSION TOMOHRAPHY (PET)
9.2.6 OTHERS
9.3 BIOPSY
9.3.1 CT-GUIDED NEEDLE BIOPSY
9.3.2 FINE NEEDLE ASPIRATION (FNA)
9.3.3 CORE NEEDLE BIOPSY
9.3.4 OTHERS
9.4 BLOOD TEST
9.4.1 LIVER FUNCTION TEST
9.4.2 TUMOR MARKER
9.4.2.1 CA 19-9 BIOMARKER TEST
9.4.2.2 CARCINOEMBROYNIC ANTIGEN (CEA) TEST
9.4.2.3 CA 50 MARKER TEST
9.4.2.4 OTHERS
9.4.3 OTHERS
9.5 GENOMIC TEST
9.6 OTHERS
10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES
10.1 OVERVIEW
10.2 STAGE IV
10.3 STAGE III
10.4 STAGE II
10.4.1 STAGE IIA
10.4.2 STAGE IIB
10.5 STAGE I
10.5.1 STAGE IA
10.5.2 STAGE IB
10.6 STAGE 0
11 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
11.1 OVERVIEW
11.2 EXOCRINE TUMORS
11.2.1 INSTRUMENT-BASED PRODUCTS
11.2.2 PLATFORM-BASED PRODUCTS
11.2.3 KITS AND REAGENTS
11.2.4 OTHER CONSUMABLES
11.3 NEUROENDOCRINE TUMORS
11.3.1 INSTRUMENT-BASED PRODUCTS
11.3.2 PLATFORM-BASED PRODUCTS
11.3.3 KITS AND REAGENTS
11.3.4 OTHER CONSUMABLES
12 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 INSTRUMENT-BASED PRODUCTS
12.2.1 IMAGING
12.2.2 BIOPSY
12.3 PLATFORM-BASED PRODUCTS
12.3.1 NEXT-GENERATION SEQUENCING
12.3.2 MICROARRAYS
12.3.3 PCR
12.3.4 OTHERS
12.4 KITS AND REAGENTS
12.4.1 CA19-9 PANCREATIC CANCER TEST KITS
12.4.1.1 ELISA TEST KITS
12.4.1.2 CASETTE TEST KITS
12.4.1.3 OTHERS
12.4.2 CEA PANCREATIC CANCER TEST KITS
12.4.2.1 ELISA TEST KITS
12.4.2.2 CASETTE TEST KITS
12.4.2.3 OTHERS
12.5 OTHER CONSUMABLES
13 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
13.1 OVERVIEW
13.2 FLUORESCENT IN SITU HYBRIDIZATION
13.3 NEXT GENERATION SEQUENCING
13.4 FLUORIMMUNOASSAY
13.5 COMPARATIVE GENOMIC HYBRIDIZATION
13.6 IMMUNOHISTOCHEMICAL
13.7 OTHERS
14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION
14.1 OVERVIEW
14.2 SCREENING
14.2.1 INSTRUMENT-BASED PRODUCTS
14.2.2 PLATFORM-BASED PRODUCTS
14.2.3 KITS AND REAGENTS
14.2.4 OTHER CONSUMABLES
14.3 DIAGNOSTIC AND PREDICTIVE
14.3.1 INSTRUMENT-BASED PRODUCTS
14.3.2 PLATFORM-BASED PRODUCTS
14.3.3 KITS AND REAGENTS
14.3.4 OTHER CONSUMABLES
14.4 PROGNOSTIC
14.4.1 INSTRUMENT-BASED PRODUCTS
14.4.2 PLATFORM-BASED PRODUCTS
14.4.3 KITS AND REAGENTS
14.4.4 OTHER CONSUMABLES
14.5 RESEARCH
14.5.1 INSTRUMENT-BASED PRODUCTS
14.5.2 PLATFORM-BASED PRODUCTS
14.5.3 KITS AND REAGENTS
14.5.4 OTHER CONSUMABLES
15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 DIAGNOSTIC CENTERS
15.4 CANCER RESEARCH CENTERS
15.5 ACADEMIC INSTITUTES
15.6 AMBULATORY SURGICAL CENTERS
15.7 OTHERS
16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.2 CANADA
17.1.3 MEXICO
18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
19 SWOT ANALYSIS
20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
20.1 CANON MEDICAL SYSTEMS CORPORATION
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENT
20.2 KONINKLIJKE PHILIPS N.V.
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 SIEMENS HEALTHCARE GMBH
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 COMPANY SHARE ANALYSIS
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENT
20.4 GRAIL
20.4.1 COMPANY PROFILE
20.4.2 COMPANY SHARE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 MYRIAD GENETICS, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 COMPANY SHARE ANALYSIS
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENT
20.6 BD
20.6.1 COMPANY SNAPSHOT
20.6.2 REVENUE ANALYSIS
20.6.3 PRODUCT PORTFOLIO
20.6.4 RECENT DEVELOPMENT
20.7 BODITECH MED INC.
20.7.1 COMPANY PROFILE
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.8 ABBOTT (2022)
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 FUJIFILM HOLDINGS AMERICA CORPORATION
20.9.1 COMPANY SNAPSHOT
20.9.2 REVENUE ANALYSIS
20.9.3 PRODUCT PORTFOLIO
20.9.4 RECENT DEVELOPMENT
20.1 ACCUBIOTECH CO., LTD.
20.10.1 COMPANY PROFILE
20.10.2 PRODUCT PORTFOLIO
20.10.3 RECENT DEVELOPMENTS
20.11 AGILENT TECHNOLOGIES, INC.
20.11.1 COMPANY PROFILE
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 CREATIVE BIOLABS.
20.12.1 COMPANY PROFILE
20.12.2 PRODUCT PORTFOLIO
20.12.3 RECENT DEVELOPMENT
20.13 CTK BIOTECH, INC.
20.13.1 COMPANY PROFILE
20.13.2 PRODUCT PORTFOLIO
20.13.3 RECENT DEVELOPMENT
20.14 DIASOURCE
20.14.1 COMPANY SNAPSHOT
20.14.2 PRODUCT PORTFOLIO
20.14.3 RECENT DEVELOPMENT
20.15 LABORATORY CORPORATION OF AMERICA HOLDINGS
20.15.1 COMPANY SNAPSHOT
20.15.2 REVENUE ANALYSIS
20.15.3 PRODUCT PORTFOLIO
20.15.4 RECENT DEVELOPMENTS
20.16 LEE BIOSCIENCE
20.16.1 COMPANY SNAPSHOT
20.16.2 PRODUCT PORTFOLIO
20.16.3 RECENT DEVELOPMENT
20.17 MERIDIAN BIOSCIENCE INC.
20.17.1 COMPANY PROFILE
20.17.2 PRODUCT PORTFOLIO
20.17.3 RECENT DEVELOPMENT
20.18 MP BIOMEDICALS.
20.18.1 COMPANY PROFILE
20.18.2 PRODUCT PORTFOLIO
20.18.3 RECENT DEVELOPMENTS
20.19 QIAGEN
20.19.1 COMPANY SNAPSHOT
20.19.2 REVENUE ANALYSIS
20.19.3 PRODUCT PORTFOLIO
20.19.4 RECENT DEVELOPMENT
20.2 SETIA SCIENTIFIC SOLUTION
20.20.1 COMPANY PROFILE
20.20.2 PRODUCT PORTFOLIO
20.20.3 RECENT DEVELOPMENTS
20.21 THERMO FISHER SCIENTIFIC INC.
20.21.1 COMPANY SNAPSHOT
20.21.2 REVENUE ANALYSIS
20.21.3 PRODUCT PORTFOLIO
20.21.4 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
Lista de Tablas
TABLE 1 APPROVED DIAGNOSTICS OF PANCREATIC CANCER
TABLE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA GENOMIC TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE IV IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE III IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA STAGE 0 IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA OTHER CONSUMABLES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA NEXT GENERATION SEQUENCING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA FLUORIMMUNOASSAY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA IMMUNOHISTOCHEMICAL IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA HOSPITALS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA DIAGNOSTIC CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA CANCER RESEARCH CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA ACADEMIC INSTITUTES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA DIRECT TENDER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA RETAIL SALES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 U.S. BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 107 U.S. STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 108 U.S. STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 109 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 110 U.S. EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 112 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 113 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 120 U.S. CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 121 U.S. CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 124 U.S. SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 126 U.S. PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 127 U.S. RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 128 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 129 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 130 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 132 CANADA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 139 CANADA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 140 CANADA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 141 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 142 CANADA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 144 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 145 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 149 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 150 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 151 CANADA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 156 CANADA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 157 CANADA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 158 CANADA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 159 CANADA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 160 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 168 MEXICO TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 169 MEXICO BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 170 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 171 MEXICO STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 172 MEXICO STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 173 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 174 MEXICO EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 176 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 177 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 178 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 179 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 180 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 182 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 183 MEXICO KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 184 MEXICO CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 185 MEXICO CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 186 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 188 MEXICO SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 189 MEXICO DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 190 MEXICO PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 191 MEXICO RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 192 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF PANCREATIC CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2022
FIGURE 19 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2022
FIGURE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2022
FIGURE 27 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 30 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2022
FIGURE 31 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 34 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2022
FIGURE 35 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 39 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 43 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 44 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 46 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 47 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 48 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 49 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 50 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: CATEGORY (2023-2030)
FIGURE 51 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.