Middle East And Africa Car T Cell Therapy Treatment Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
16.22 Million
USD
138.11 Million
2024
2032
USD
16.22 Million
USD
138.11 Million
2024
2032
| 2025 –2032 | |
| USD 16.22 Million | |
| USD 138.11 Million | |
|
|
|
|
Segmentación del mercado de tratamientos de terapia celular CAR-T de MEA, por producto (células CAR-T autólogas y células CAR-T alogénicas), estructura (células CAR-T de primera generación, células CAR-T de segunda generación, células CAR-T de tercera generación y células CAR-T de cuarta generación), antígenos dirigidos (antígenos en tumores sólidos, antígenos en neoplasias hematológicas y otros), marca (Yescarta, Kymriah, Tecartus y otros), aplicación terapéutica (linfoma difuso de células B grandes, linfoma folicular, leucemia linfoblástica aguda (LLA), linfoma de células del manto, mieloma múltiple, neoplasias hematológicas, cáncer de pulmón, leucemia linfocítica crónica, cáncer gástrico, cáncer de páncreas, cáncer de mama y otros), usuario final (hospitales, clínicas especializadas y otros), canal de distribución (hospitales, farmacias y otros): tendencias de la industria y pronóstico 2032
Tamaño del mercado de tratamientos con terapia celular CAR-T en MEA
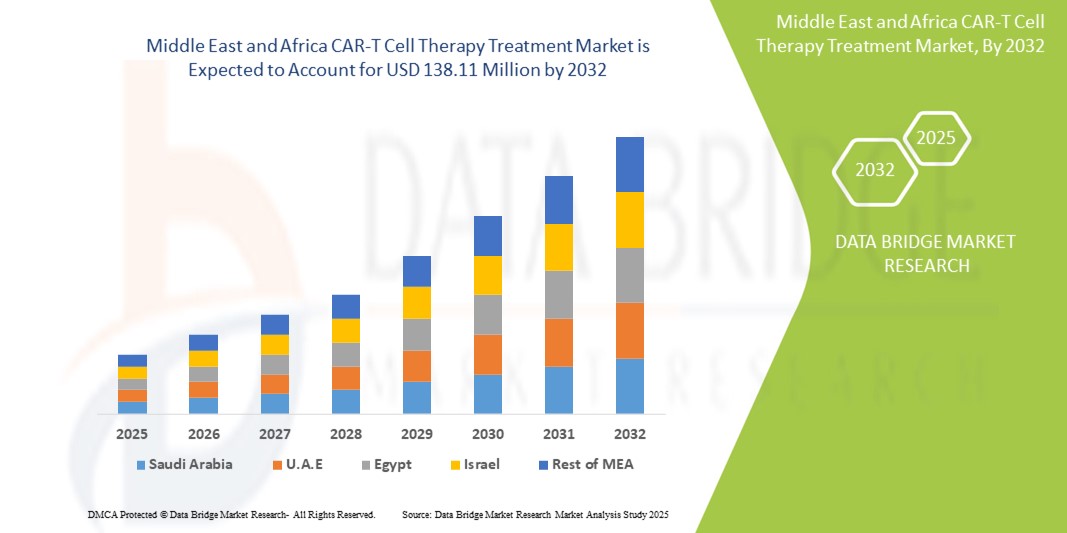
- El tamaño del mercado de tratamiento de terapia con células CAR-T de MEA se valoró en USD 16,22 millones en 2024 y se espera que alcance los USD 138,11 millones para 2032 , con una CAGR del 30,7% durante el período de pronóstico.
- El crecimiento del mercado se debe en gran medida a la creciente prevalencia de neoplasias hematológicas y a la progresiva expansión de la infraestructura de terapia celular y génica avanzada en la región de Oriente Medio y África (MEA). La mejora de los marcos regulatorios, la creciente inversión en biotecnología y el establecimiento de centros de investigación y fabricación de CAR-T están acelerando el desarrollo clínico y la disponibilidad de las terapias con células CAR-T.
- Además, la creciente demanda de opciones de tratamiento dirigidas, personalizadas y altamente efectivas por parte de los pacientes está posicionando la terapia con células CAR-T como un enfoque innovador en el tratamiento del cáncer. Estos factores convergentes están acelerando la adopción de las soluciones de tratamiento con células CAR-T en Oriente Medio y África, impulsando así significativamente el crecimiento de la industria en mercados clave como los Emiratos Árabes Unidos, Arabia Saudita, Sudáfrica y Egipto.
Análisis del mercado de tratamientos con terapia celular CAR-T en MEA
- La terapia con células CAR-T, una inmunoterapia innovadora que reprograma las células T de un paciente para reconocer y atacar las células cancerosas, se está convirtiendo cada vez más en un enfoque de tratamiento crítico para las neoplasias hematológicas en la región MEA debido a su alta eficacia, naturaleza personalizada y capacidad para inducir remisiones duraderas en casos recidivantes o refractarios.
- La creciente demanda de terapia con células CAR-T se ve impulsada principalmente por la creciente prevalencia de cánceres de la sangre, como la leucemia y el linfoma, la creciente conciencia entre los proveedores de atención médica y los pacientes, y la expansión de la investigación clínica en la región MEA.
- Los Emiratos Árabes Unidos dominaron el mercado de tratamiento con terapia celular CAR-T en Oriente Medio y África con la mayor participación en los ingresos del 26,8 % en 2024, caracterizado por una sólida infraestructura de atención médica, la adopción temprana de tratamientos innovadores y una inversión significativa en terapias personalizadas contra el cáncer.
- Se espera que Israel sea el país de más rápido crecimiento en el mercado de tratamiento de terapia celular CAR-T de MEA con una CAGR proyectada del 17,5 % entre 2025 y 2032, impulsado por una investigación de vanguardia en inmunooncología, un ecosistema biotecnológico en expansión y una alta actividad de ensayos clínicos en terapia celular y génica.
- El segmento de antígenos en neoplasias hematológicas dominó el mercado de tratamiento con terapia celular CAR-T de MEA con una participación de mercado del 66,4 % en 2024, impulsado por el éxito de las terapias CAR-T para cánceres de la sangre como leucemia, linfoma y mieloma múltiple.
Alcance del informe y segmentación del mercado de tratamiento con terapia celular CAR-T de MEA
|
Atributos |
Perspectivas clave del mercado del tratamiento con terapia celular CAR-T en MEA |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Oriente Medio y África
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de tratamientos con terapia celular CAR-T en MEA
Creciente acceso a inmunoterapias avanzadas en Oriente Medio y África
- Una tendencia significativa y en auge en el mercado de la terapia celular CAR-T en Oriente Medio y África es el creciente acceso a inmunoterapias avanzadas en toda la región, especialmente en centros de salud urbanos. Hospitales y clínicas especializadas en Oriente Medio y algunas partes de África están adoptando cada vez más terapias celulares contra el cáncer, lo que refleja una transición hacia la medicina de precisión.
- Por ejemplo, varios centros oncológicos en países como los Emiratos Árabes Unidos (EAU) y Arabia Saudita se han asociado con empresas biotecnológicas internacionales para explorar ensayos clínicos y protocolos de tratamiento con CAR-T. Estas colaboraciones permiten a los médicos locales ofrecer terapias de vanguardia que antes estaban limitadas a los mercados occidentales.
- La creciente conciencia regional sobre los tratamientos oncológicos personalizados está impulsando a los proveedores de atención médica, tanto públicos como privados, a incorporar las terapias CAR-T en las estrategias de atención oncológica a largo plazo. El aumento de las campañas de apoyo y concienciación de los pacientes sobre las neoplasias hematológicas también contribuye al crecimiento del mercado.
- Además, la región MEA está experimentando un aumento de las iniciativas gubernamentales destinadas a mejorar las tasas de supervivencia al cáncer, como los programas nacionales de control del cáncer y las infraestructuras para las pruebas genómicas del cáncer. Estos esfuerzos son cruciales para identificar a los pacientes elegibles para la terapia con células CAR-T y optimizar el acceso al tratamiento.
- Los centros médicos académicos en países como Sudáfrica y Egipto ahora están explorando modelos de asociación con compañías biofarmacéuticas multinacionales para establecer centros de investigación y fabricación de CAR-T, impulsando aún más la accesibilidad regional de la terapia.
- La convergencia de la creciente incidencia del cáncer, el desarrollo de la infraestructura médica y el apoyo regulatorio está creando un entorno favorable para la expansión del mercado de la terapia CAR-T en Oriente Medio y África.
Dinámica del mercado de tratamientos con terapia celular CAR-T en MEA
Conductor
Necesidad creciente debido al aumento de la incidencia del cáncer y los avances en la medicina de precisión.
- La creciente prevalencia de neoplasias hematológicas, como el linfoma difuso de células B grandes, el mieloma múltiple y la leucemia linfoblástica aguda en Oriente Medio y África (MEA), es un factor clave para la creciente demanda de terapias con células CAR-T. El aumento de la incidencia del cáncer y la necesidad insatisfecha de tratamientos eficaces y duraderos impulsan a los sistemas sanitarios a adoptar inmunoterapias de vanguardia.
- Por ejemplo, en marzo de 2024, proveedores regionales de atención médica en Arabia Saudita y los Emiratos Árabes Unidos colaboraron con empresas biotecnológicas globales para establecer centros de terapia CAR-T, con el objetivo de mejorar la accesibilidad y los resultados de los pacientes. Estas iniciativas reflejan el compromiso estratégico de la región de integrar terapias celulares avanzadas en las vías nacionales de atención oncológica.
- Además, la región MEA está experimentando un aumento de las inversiones en medicina personalizada, lo que apoya la aplicación de terapias CAR-T adaptadas a los perfiles tumorales de cada paciente. La disponibilidad de productos CAR-T autólogos como Yescarta y Kymriah está contribuyendo a su adopción temprana en hospitales especializados e institutos de investigación.
- La expansión de los presupuestos gubernamentales para la salud y los marcos regulatorios favorables, especialmente en los países del CCG, están facilitando los ensayos clínicos y las aprobaciones comerciales de los productos CAR-T. La creciente concienciación entre oncólogos y pacientes sobre la eficacia de estas terapias en casos de recaída o refractarios está impulsando aún más la expansión del mercado.
Restricción/Desafío
“ Altos costos de tratamiento e infraestructura limitada en los mercados emergentes ”
- Uno de los desafíos más críticos que frena el mercado de la terapia celular CAR-T en MEA es el alto costo de la terapia, que puede oscilar entre USD 350.000 y USD 500.000 por ciclo de tratamiento. Este costo incluye la recolección de células, la fabricación, la atención hospitalaria y el seguimiento, lo que supone una carga significativa para los sistemas de salud públicos y los pacientes sin seguro médico.
- Por ejemplo, varios países africanos carecen actualmente de infraestructura para la fabricación y el almacenamiento de células CAR-T, lo que genera una dependencia de la logística internacional y plazos de entrega prolongados. Esto afecta la administración oportuna de la terapia y compromete los resultados, especialmente en cánceres agresivos.
- Además, la escasez de personal clínico capacitado e instalaciones certificadas para administrar la terapia CAR-T obstaculiza el acceso generalizado. La limitada capacidad diagnóstica para identificar a los pacientes elegibles retrasa aún más el inicio del tratamiento.
- Para superar estos obstáculos, los actores del mercado se están centrando en el desarrollo de unidades de fabricación descentralizadas de CAR-T y en el establecimiento de programas de capacitación en colaboración con los gobiernos regionales. Los esfuerzos para reducir los costos de producción mediante plataformas de células CAR-T alogénicas (listas para usar) también podrían ampliar la asequibilidad en los países de ingresos bajos y medios de la región MEA.
- Abordar los desafíos de reembolso, las complejidades regulatorias y las limitaciones logísticas a través de asociaciones regionales y modelos de precios innovadores será vital para el éxito a largo plazo del mercado de tratamiento de terapia con células CAR-T de MEA.
Alcance del mercado de tratamientos con terapia celular CAR-T de MEA
El mercado de tratamiento con terapia con células CAR-T de MEA está segmentado en cuatro segmentos notables según el producto, la estructura, los antígenos objetivo y la aplicación terapéutica.
• Por producto
En cuanto al producto, el mercado de terapia con células CAR-T en MEA se segmenta en células CAR-T autólogas y células CAR-T alogénicas. El segmento de células CAR-T autólogas dominó el mercado con la mayor participación en los ingresos, con un 72,3 % en 2024, gracias a una menor inmunogenicidad y a las ventajas de personalización.
Se espera que el segmento de células CAR-T alogénicas experimente la CAGR más rápida del 24,8 % entre 2025 y 2032 debido a su disponibilidad lista para usar y su relación costo-beneficio.
• Por Estructura
Según su estructura, el mercado de terapia con células CAR-T en MEA se segmenta en células CAR-T de primera generación, células CAR-T de segunda generación, células CAR-T de tercera generación y células CAR-T de cuarta generación. El segmento de células CAR-T de segunda generación tuvo la mayor participación de mercado, con un 58,9 %, en 2024, gracias a su mayor eficacia y amplia aplicación clínica.
Se espera que el segmento de células CAR-T de cuarta generación crezca a la CAGR más rápida del 26,1 % durante el período de pronóstico, impulsado por tecnologías avanzadas de edición genética y capacidades de múltiples objetivos.
• Por antígenos específicos
En función de los antígenos diana, el mercado de terapia con células CAR-T en MEA se segmenta en antígenos para tumores sólidos, antígenos para neoplasias hematológicas y otros. Los antígenos para neoplasias hematológicas representaron la mayor participación en los ingresos, con un 66,4 %, en 2024, impulsados por el éxito de las terapias CAR-T para cánceres hematológicos.
Se proyecta que los antígenos en los tumores sólidos crecerán a la CAGR más alta del 25,7 % durante el período de pronóstico, impulsado por los ensayos clínicos en curso y las innovaciones en la focalización del microambiente tumoral.
• Por marca
Según la marca, el mercado de terapia con células CAR-T de MEA se segmenta en yescarta, kymriah, tecartus y otros. Yescarta tuvo la mayor participación de mercado, con un 41,2 % en 2024, con una fuerte adopción para el linfoma difuso de células B grandes (LDCBG) y otros linfomas.
Se anticipa que Tecartus registrará la CAGR más rápida del 22,9 % entre 2025 y 2032, impulsada por su desempeño en el linfoma de células del manto.
• Por aplicación terapéutica
En función de su aplicación terapéutica, el mercado de la terapia con células CAR-T de MEA se segmenta en linfoma difuso de células B grandes, linfoma folicular, leucemia linfoblástica aguda (LLA), linfoma de células del manto, mieloma múltiple, neoplasias hematológicas, cáncer de pulmón, leucemia linfocítica crónica, cáncer gástrico, cáncer de páncreas, cáncer de mama, entre otros. El linfoma difuso de células B grandes (LDCBG) obtuvo la mayor participación, con un 36,5 %, en 2024, gracias a las aprobaciones tempranas y los resultados satisfactorios en los pacientes.
Se espera que el mieloma múltiple crezca a la CAGR más rápida del 28,3 % durante el período de pronóstico, impulsado por los nuevos lanzamientos de productos CAR-T dirigidos a BCMA en MEA.
• Por el usuario final
En función del usuario final, el mercado de terapia con células CAR-T en MEA se segmenta en hospitales, clínicas especializadas y otros. Los hospitales lideraron el segmento con una participación de mercado del 69,8 % en 2024 gracias a su capacidad para respaldar los protocolos de infusión, monitorización y recuperación de células CAR-T.
Se espera que las clínicas especializadas crezcan a la CAGR más alta del 21,6 % durante el período de pronóstico, beneficiándose de la descentralización y la expansión de las redes de inmunoterapia.
• Por canal de distribución
Según el canal de distribución, el mercado de terapia con células CAR-T de MEA se segmenta en farmacia hospitalaria y otros. El segmento de farmacia hospitalaria tuvo la mayor participación de mercado, con un 78,1 %, en 2024, gracias a la complejidad de la cadena de frío y los requisitos de cumplimiento normativo.
Se proyecta que el segmento otros se expandirá a una CAGR del 19,5 % durante el período de pronóstico, a medida que más fabricantes de CAR-T agilizan la distribución a través de proveedores de logística especializados.
Análisis regional del mercado de terapia con células CAR-T en MEA
- Los Emiratos Árabes Unidos dominaron el mercado de tratamiento con terapia celular CAR-T en Oriente Medio y África con la mayor participación en los ingresos del 26,8 % en 2024, caracterizado por una sólida infraestructura de atención médica, la adopción temprana de tratamientos innovadores y una inversión significativa en terapias personalizadas contra el cáncer.
- Los pacientes y los proveedores de atención médica de la región valoran cada vez más la eficacia específica, el enfoque personalizado y el potencial de remisión a largo plazo que ofrecen las terapias con células CAR-T en el tratamiento de cánceres hematológicos como la leucemia y el linfoma.
- Esta creciente adopción se ve impulsada además por la expansión de la actividad de ensayos clínicos, la mejora del acceso a la atención oncológica avanzada y las crecientes inversiones gubernamentales en tratamientos basados en células, lo que posiciona a la terapia CAR-T como una opción transformadora en mercados selectos de MEA.
Análisis del mercado de terapia con células CAR-T en Arabia Saudita (MEA)
El mercado de tratamientos con terapia celular CAR-T en Arabia Saudita representó el 21,3 % de los ingresos del mercado de Oriente Medio y África en 2024 y se prevé que crezca a una tasa de crecimiento anual compuesta (TCAC) sustancial durante el período de pronóstico. Este crecimiento está impulsado por la estrategia de transformación sanitaria Visión 2030 del gobierno, la expansión de centros oncológicos especializados y las alianzas con empresas biotecnológicas internacionales. El aumento de casos de cáncer hematológico y el impulso nacional a la adopción de la medicina de precisión están impulsando la expansión del mercado.
Perspectiva del mercado de tratamientos con terapia celular CAR-T en los EAU MEA
El mercado de terapia con células CAR-T en los EAU obtuvo la mayor cuota de mercado en ingresos, con un 26,8 %, en la región MEA en 2024, gracias a su sólida inversión en terapias avanzadas, el alto gasto sanitario y la proactiva gestión de las aprobaciones regulatorias. Las terapias CAR-T se están adoptando rápidamente en los principales hospitales oncológicos del país, gracias a la colaboración con empresas innovadoras globales como Gilead y Novartis.
Perspectiva del mercado de tratamientos con células CAR-T en Israel MEA
El mercado israelí de terapia con células CAR-T presenta una tasa de crecimiento anual compuesta (TCAC) proyectada del 17,5 % entre 2025 y 2032, gracias a su avanzado sector biotecnológico, su sólida capacidad académica en I+D y su alta actividad en ensayos clínicos con células CAR-T. El mercado está a punto de expandirse aún más gracias al creciente desarrollo y aplicación nacional de inmunoterapias de última generación.
Cuota de mercado de tratamientos de terapia con células CAR-T en MEA
La industria del tratamiento con terapia celular CAR-T de MEA está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Novartis AG (Suiza)
- Gilead Sciences, Inc. (EE. UU.)
- Bristol-Myers Squibb Company (EE. UU.)
- Johnson & Johnson Services, Inc. (EE. UU.)
- Autolus Therapeutics plc (Reino Unido)
- Poseida Therapeutics, Inc. (EE. UU.)
- Sorrento Therapeutics, Inc. (EE. UU.)
- Miltenyi Biotec (Alemania)
- Terapéutica CARsgen (China)
- JW Therapeutics (Shanghái) Co., Ltd. (China)
- Corporación Biotecnológica Legend (China)
- Tessa Therapeutics (Singapur)
- Adaptimmune Therapeutics plc (Reino Unido)
- Bluebird Bio, Inc. (EE. UU.)
- Celyad Oncology SA (Bélgica)
- Terapia alogénica (EE. UU.)
- Immatics NV (Alemania)
- Pfizer Inc. (EE. UU.)
Últimos avances en el mercado de tratamientos con terapia celular CAR-T de MEA
- En diciembre de 2023, Gilead Sciences, Inc. anunció que la FDA de EE. UU. aprobó una actualización de la etiqueta para Yescarta (axicabtagene ciloleucel) para incluir el análisis primario de supervivencia general (SG) del estudio emblemático de fase 3 ZUMA-7 que muestra una mejora estadísticamente significativa para Yescarta en la SG en comparación con el estándar de atención (SOC) como tratamiento de segunda línea con intención curativa para pacientes con linfoma de células B grandes recidivante o refractario (LBCL R/R) dentro de los 12 meses posteriores a la finalización de la terapia de primera línea.
- En mayo de 2022, Bristol-Myers Squibb Company anunció la aprobación de Opdivo más Yervoy como tratamiento de primera línea para pacientes adultos por parte del Ministerio de Salud, Trabajo y Bienestar Social de Japón. Esto podría ayudar a la compañía a fortalecer su cartera de productos.
- En febrero de 2022, la FDA aprobó CARVYKTI (ciltacabtagene autoleucel) de Janssen para el tratamiento de adultos con mieloma múltiple en recaída o refractario tras cuatro o más líneas de tratamiento previas, lo que marca la primera terapia celular de Janssen. Esto subrayará la dedicación de Janssen para avanzar en sus opciones de tratamiento oncológico.
- En diciembre de 2021, Novartis AG firmó un acuerdo con BeiGene, Ltd. para ociperlimab (BGB-A1217), lo que refuerza la investigación y el desarrollo en inmunooncología de la compañía. Esta colaboración contribuye a la iniciativa más amplia de Novartis Oncology para impulsar la innovación en tratamientos contra el cáncer mediante la incorporación de una terapia potencialmente transformadora a su plataforma de inmunoterapia en expansión.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.