Middle East And Africa Adalimumab Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
134.81 Million
USD
181.68 Million
2021
2029
USD
134.81 Million
USD
181.68 Million
2021
2029
| 2022 –2029 | |
| USD 134.81 Million | |
| USD 181.68 Million | |
|
|
|
Mercado de adalimumab en Oriente Medio y África, por clase de fármaco (antirreumáticos, inhibidores del TNF alfa, otros), indicación ( artritis reumatoide , espondilitis anquilosante, psoriasis en placas crónica, enfermedad de Crohn , colitis ulcerosa, artritis psoriásica, artritis idiopática juvenil, hidradenitis supurativa, intermedio no infeccioso, otros), tipo (biológicos, biosimilares), dosis (40 mg/0,4 mlg, 80 mg/0,8 mlg, 20 mg/0,2 mlg, 10 mg/0,1 mlg, otros), tipo de fármaco (de marca, genéricos), vía de administración (oral, parenteral, otros), grupo de edad (pediátrico, adulto, geriátrico), forma farmacéutica (comprimido, inyección, solución, otros), usuarios finales (hospitales, clínicas especializadas, atención domiciliaria, Otros), Canal de distribución (farmacia hospitalaria, farmacia minorista, farmacia en línea, otros): tendencias de la industria y pronóstico hasta 2029
Análisis y tamaño del mercado
El adalimumab, que se autorizó por primera vez en los Estados Unidos, ahora está disponible en más de 60 países. Su mercado en Oriente Medio y África está consolidado, y sólo unas pocas empresas intentan superar a otras en cuanto a precio. La mayoría de los principales actores están concentrando actualmente sus esfuerzos en el desarrollo de biosimilares de adalimumab para el tratamiento de la artritis reumatoide y la psoriasis. Esto se ve en los ensayos clínicos que prueban la seguridad y eficacia de los biosimilares de adalimumab en el tratamiento de trastornos médicos. Muchos trastornos inflamatorios en adultos se tratan con adalimumab, entre ellos la colitis ulcerosa, la artritis reumatoide, la artritis psoriásica, la espondilitis anquilosante, la psoriasis en placas y la hidradenitis supurativa.
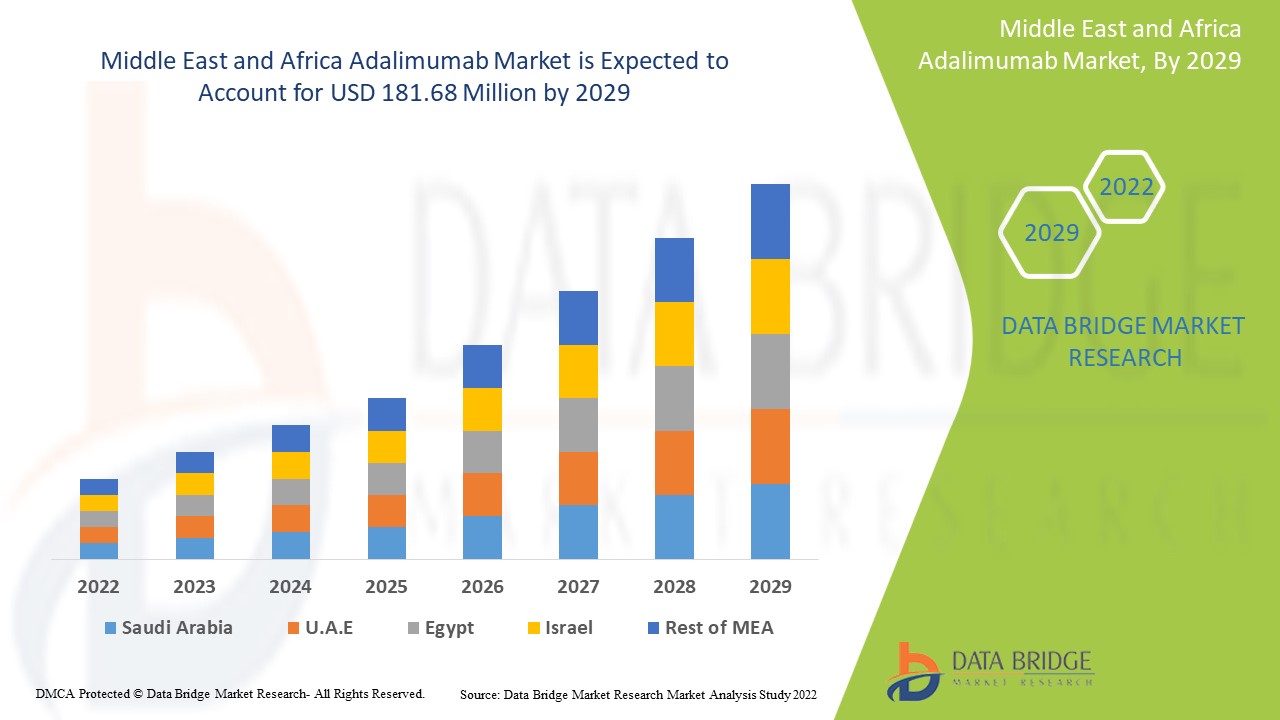
Data Bridge Market Research analiza que el mercado de adalimumab en Oriente Medio y África se valoró en USD 134,81 millones en 2021 y se espera que alcance los USD 181,68 millones para 2029, registrando una CAGR del 3,80% durante el período de pronóstico de 2022 a 2029. Además de los conocimientos del mercado, como el valor de mercado, la tasa de crecimiento, los segmentos del mercado, la cobertura geográfica, los actores del mercado y el escenario del mercado, el informe de mercado curado por el equipo de Data Bridge Market Research también incluye un análisis de expertos en profundidad, epidemiología del paciente, análisis de la cartera, análisis de precios y marco regulatorio.
Alcance del informe y segmentación del mercado
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2022 a 2029 |
|
Año base |
2021 |
|
Años históricos |
2020 (Personalizable para 2014 - 2019) |
|
Unidades cuantitativas |
Ingresos en millones de USD, volúmenes en unidades, precios en USD |
|
Segmentos cubiertos |
Clase de fármaco (antirreumáticos, inhibidores del TNF alfa, otros), indicación (artritis reumatoide, espondilitis anquilosante, psoriasis en placas crónica, enfermedad de Crohn, colitis ulcerosa, artritis psoriásica, artritis idiopática juvenil, hidradenitis supurativa, intermedio no infeccioso, otros), tipo (biológicos, biosimilares), dosis (40 mg/0,4 mlg, 80 mg/0,8 mlg, 20 mg/0,2 mlg, 10 mg/0,1 mlg, otros), tipo de fármaco (de marca, genéricos), vía de administración (oral, parenteral, otros), grupo de edad (pediátrico, adulto, geriátrico), forma farmacéutica (comprimido, inyección, solución, otros), usuarios finales (hospitales, clínicas especializadas, atención domiciliaria, otros), canal de distribución (farmacia hospitalaria, venta minorista) Farmacia, Farmacia Online, Otros) |
|
Países cubiertos |
Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Resto de Medio Oriente y África (MEA) como parte de Medio Oriente y África (MEA). |
|
Actores del mercado cubiertos |
Mylan NV (EE. UU.), AbbVie Inc. (EE. UU.), Zydus Cadila (India), Pfizer Inc. (EE. UU.), Hetero Biopharma Ltd. (India), Boehringer Ingelheim International GmbH. (Alemania) |
|
Oportunidades de mercado |
|
Definición de mercado
El adalimumab es un medicamento de venta con receta que se vende bajo las marcas Humira y Exemptia. La artritis reumatoide, la artritis psoriásica, la enfermedad de Crohn, la psoriasis y la colitis ulcerosa se tratan con adalimumab. El TNF (factor de necrosis tumoral alfa) se une habitualmente al adalimumab. Cuando el TNF interactúa con los receptores TBF, se desencadena una respuesta inflamatoria a la enfermedad autoinmune. Al unirse al TNF, el adalimumab reduce la probabilidad de una respuesta inflamatoria.
Dinámica del mercado de adalimumab en Oriente Medio y África
Conductores
- Aumento de la tasa de incidencia de enfermedades autoinmunes
Se prevé que el aumento de la incidencia de enfermedades autoinmunes como la artritis psoriásica, la psoriasis en placas, la colitis ulcerosa, la espondilitis anquilosante, la artritis reumatoide y la enfermedad de Crohn aumente la tasa de crecimiento del mercado. Adalimumab es un medicamento que reduce el dolor y la hinchazón, al mismo tiempo que ralentiza la progresión de la artritis. Adalimumab se utiliza para tratar la artritis relacionada con la entesitis activa, la artritis reumatoide, la osteoartritis, la artritis idiopática juvenil poliarticular y otras enfermedades autoinmunes. Junto con esto, la creciente prevalencia de trastornos crónicos aumentará la demanda del mercado de adalimumab.
- Aumentar la inversión en infraestructura sanitaria
Otro factor importante que influye en la tasa de crecimiento del mercado de adalimumab es el aumento del gasto sanitario, que contribuye a mejorar su infraestructura. Además, varias organizaciones gubernamentales tienen como objetivo mejorar la infraestructura sanitaria aumentando la financiación, lo que influirá aún más en la dinámica del mercado.
- Aumento de la incidencia de trastornos de la piel
Se estima que la incidencia de los trastornos de la piel impulsará la tasa de crecimiento del mercado durante el período de pronóstico de 2022 a 2029. La Organización Mundial de la Salud (OMS) estima que 900 millones de personas en todo el mundo padecen enfermedades de la piel en un momento dado. El TNF-alfa (factor de necrosis tumoral alfa) es un participante fundamental en el proceso inflamatorio que causa trastornos de la piel, incluida la psoriasis. El adalimumab se dirige a esta proteína en el cuerpo. La psoriasis es una afección cutánea que desarrolla manchas rojas escamosas en las rodillas, los codos, el tronco y el cuero cabelludo. La psoriasis es causada por una respuesta hiperactiva del sistema inmunológico, que es suprimida por el adalimumab. Según la National Psoriasis Foundation, 125 millones de personas en todo el mundo padecen psoriasis, lo que representa entre el 2 y el 3 por ciento de la población general, lo que impulsa el crecimiento del mercado.
Además, las crecientes iniciativas de organizaciones públicas y privadas para difundir la información y la creciente demanda de medicamentos biosimilares debido a su relación coste-eficacia ampliarán el mercado de adalimumab. Además, el aumento de la población geriátrica y de los casos de infección de las vías respiratorias superiores darán lugar a la expansión del mercado de adalimumab.
Oportunidades
- Aumento del número de actividades de investigación y desarrollo
Además, el crecimiento del mercado se ve impulsado por un aumento en el número de actividades de investigación y desarrollo, lo que brindará oportunidades beneficiosas para el crecimiento del mercado de adalimumab. Junto con esto, el aumento de las aprobaciones y lanzamientos de medicamentos impulsará aún más la tasa de crecimiento del mercado.
Además, la creciente inversión para el desarrollo de tecnologías avanzadas y el aumento del número de mercados emergentes brindarán más oportunidades beneficiosas para el crecimiento del mercado de adalimumab durante el período de pronóstico.
Restricciones/Desafíos
- Alto costo y efectos secundarios asociados con adalimumab
El adalimumab es bastante caro para las personas de países de ingresos bajos y medios, ya que cuesta aproximadamente entre 2000 y 3000 dólares cada infusión. Además, se espera que los efectos negativos del adalimumab limiten la expansión del mercado. Fiebre, inflamación de los ganglios linfáticos, sudores nocturnos, sensación general de malestar, dolor en las articulaciones y los músculos, sarpullido, aparición de hematomas o sangrado con facilidad y otros son algunos de los efectos adversos frecuentes del adalimumab. El adalimumab también puede causar un tipo de linfoma que es mortal, así como cánceres de hígado, bazo y médula ósea. Esto es más común en adolescentes y hombres jóvenes con enfermedad de Crohn o colitis ulcerosa, lo que frena el crecimiento del mercado.
Por otra parte, la falta de infraestructura sanitaria en las economías en desarrollo y el estricto proceso regulatorio vinculado con la aprobación de productos biosimilares desafiarán el mercado de adalimumab. Además, la expiración de las patentes de los medicamentos actuará como un freno y obstaculizará aún más la tasa de crecimiento del mercado durante el período previsto de 2022 a 2029.
Este informe sobre el mercado de adalimumab en Oriente Medio y África proporciona detalles de los nuevos desarrollos recientes, regulaciones comerciales, análisis de importación y exportación, análisis de producción, optimización de la cadena de valor, participación de mercado, impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, análisis estratégico del crecimiento del mercado, tamaño del mercado, crecimientos del mercado por categorías, nichos de aplicación y dominio, aprobaciones de productos, lanzamientos de productos, expansiones geográficas, innovaciones tecnológicas en el mercado. Para obtener más información sobre el mercado de adalimumab en Oriente Medio y África, comuníquese con Data Bridge Market Research para obtener un informe de analista; nuestro equipo lo ayudará a tomar una decisión de mercado informada para lograr el crecimiento del mercado.
Análisis de la epidemiología de los pacientes
El mercado de adalimumab en Oriente Medio y África también le proporciona un análisis detallado del mercado para el análisis de pacientes, el pronóstico y las curas. La prevalencia, la incidencia, la mortalidad y las tasas de adherencia son algunas de las variables de datos que están disponibles en el informe. Se analizan los análisis de impacto directo o indirecto de la epidemiología en el crecimiento del mercado para crear un modelo estadístico multivariante de cohorte más sólido para pronosticar el mercado en el período de crecimiento.
Impacto de la COVID-19 en el mercado de adalimumab en Oriente Medio y África
Desde su aparición en diciembre de 2019, el virus COVID-19 se ha propagado a casi todos los países del planeta, lo que llevó a la Organización Mundial de la Salud (OMS) a declararlo una emergencia de salud pública. El COVID-19, un nuevo coronavirus, fue identificado como el agente causal de los casos de neumonía. Este virus se propagó rápidamente por todo el mundo y mató a un gran número de personas. La Organización Mundial de la Salud (OMS) calificó al COVID-19 como una pandemia en Oriente Medio y África en marzo de 2020, y recomendó medidas rigurosas para prevenir la propagación de la enfermedad. Desde entonces, la pandemia ha retrasado la expansión del sector sanitario y ha interrumpido la cadena de suministro. Además, los gobiernos de varios países han impuesto confinamientos a nivel nacional para detener la propagación del COVID-19. Del mismo modo, las organizaciones sanitarias de numerosos países de todo el mundo estaban teniendo dificultades para continuar con sus actividades de la cadena de suministro. El mercado de adalimumab se vio obstaculizado por la lentitud de la cadena de suministro.
Desarrollo reciente
- En octubre de 2021, la Administración de Alimentos y Medicamentos de los Estados Unidos (FDA) anunció la aprobación del primer producto biosimilar intercambiable para el tratamiento de diversas enfermedades inflamatorias. La vía de aprobación de biosimilares e intercambiables se estableció para ayudar a los pacientes con afecciones médicas críticas a tener acceso a más opciones de tratamiento. Cyltezo es el primer anticuerpo monoclonal intercambiable y el segundo medicamento biosimilar intercambiable autorizado por la FDA.
Alcance del mercado de adalimumab en Oriente Medio y África
El mercado de adalimumab en Oriente Medio y África está segmentado en función de la clase de fármaco, el tipo, la indicación, la forma farmacéutica, la potencia de la dosis, el tipo de fármaco, la vía de administración, el grupo de edad, los usuarios finales y el canal de distribución. El crecimiento entre estos segmentos le ayudará a analizar los escasos segmentos de crecimiento de las industrias y proporcionará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Clase de droga
- Antirreumáticos
- Inhibidores del TNF alfa
- Otros
Indicación
- Artritis reumatoide
- Espondiloartritis anquilosante
- Psoriasis crónica en placas
- Enfermedad de Crohn
- Colitis ulcerosa
- Artritis psoriásica
- Artritis reumatoide juvenil idiopática
- Hidradenitis supurativa
- Intermedio no infeccioso
- Otros
Tipo
- Biológicos
- Biosimilares
Dosis de fuerza
- 40 mg/0,4 ml/g
- 80 mg/0,8 ml/g
- 20 mg/0,2 ml/g
- 10 mg/0,1 ml/g
- Otros
Tipo de droga
- De marca
- Genéricos
Vía de administración
- Oral
- Parenteral
- Otros
Forma de dosificación
- Inyección
- Solución
- Tableta
- Otros
Grupo de edad
- Pediátrico
- Adulto
- Geriátrico
Usuarios finales
- Hospitales
- Clínicas de especialidades
- Cuidado domiciliario
- Otros
Canal de distribución
- Farmacia hospitalaria
- Farmacia minorista
- Farmacia en línea
- Otros
Análisis y perspectivas regionales del mercado de adalimumab en Oriente Medio y África
Se analiza el mercado de adalimumab en Oriente Medio y África y se proporcionan información y tendencias del tamaño del mercado por país, clase de fármaco, tipo, indicación, forma de dosificación, concentración de la dosis, tipo de fármaco, vía de administración, grupo de edad, usuarios finales y canal de distribución como se menciona anteriormente.
Los países cubiertos en el informe del mercado de adalimumab en Medio Oriente y África son Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, el resto de Medio Oriente y África (MEA) como parte de Medio Oriente y África (MEA).
Arabia Saudita domina el mercado de adalimumab debido al creciente número de actividades de investigación y desarrollo para superar la carga de enfermedades artríticas en esta región.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Puntos de datos como análisis de la cadena de valor aguas abajo y aguas arriba, tendencias técnicas y análisis de las cinco fuerzas de Porter, estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de Medio Oriente y África y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales al proporcionar un análisis de pronóstico de los datos del país.
Panorama competitivo y análisis de la cuota de mercado de adalimumab en Oriente Medio y África
El panorama competitivo del mercado de adalimumab en Oriente Medio y África proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia en Oriente Medio y África, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y la extensión de los productos, el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado de adalimumab en Oriente Medio y África.
Algunos de los principales actores que operan en el mercado de adalimumab en Oriente Medio y África son:
- Mylan NV (Estados Unidos)
- Zydus Cadila (India)
- Boehringer Ingelheim International GmbH (Alemania)
- AbbVie Inc. (Estados Unidos)
- Abbott (Estados Unidos)
- Hetero Biopharma Ltd. (India)
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2 MG/0.4ML
13.3 MG/0.8ML
13.4 MG/0.4ML
13.5 MG/0.1ML
13.6 OTHERS
14 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 MIDDLE EAST & AFRICA
19.1.1 SAUDI ARABIA
19.1.2 SOUTH AFRICA
19.1.3 UAE
19.1.4 ISRAEL
19.1.5 KUWAIT
19.1.6 EGYPT
19.1.7 REST OF MIDDLE EAST & AFRICA
20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
Lista de Tablas
LIST OF TABLES
TABLE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 SAUDI ARABIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 SAUDI ARABIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 SAUDI ARABIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 SAUDI ARABIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 SAUDI ARABIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 SAUDI ARABIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 SAUDI ARABIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 SAUDI ARABIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 SAUDI ARABIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 SAUDI ARABIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 SOUTH AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 SOUTH AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 SOUTH AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 SOUTH AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 SOUTH AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 SOUTH AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 SOUTH AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 SOUTH AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 SOUTH AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 SOUTH AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 UAE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 UAE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 UAE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 UAE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 UAE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 UAE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 UAE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 UAE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 UAE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 UAE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 ISRAEL ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 ISRAEL ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 ISRAEL BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 ISRAEL ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 ISRAEL ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 ISRAEL GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 ISRAEL ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 ISRAEL ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 ISRAEL ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 ISRAEL ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 KUWAIT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 KUWAIT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 KUWAITBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 KUWAIT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 KUWAIT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 KUWAIT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 KUWAIT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 KUWAIT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 KUWAIT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 KUWAIT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 EGYPT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 EGYPT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 EGYPT BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 EGYPT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 EGYPT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 EGYPT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 EGYPT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 EGYPT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 EGYPT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 EGYPT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 REST OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
Lista de figuras
LIST OF FIGURES
FIGURE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.