Global Vaccine Adjuvants Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.73 Billion
USD
4.74 Billion
2024
2032
USD
1.73 Billion
USD
4.74 Billion
2024
2032
| 2025 –2032 | |
| USD 1.73 Billion | |
| USD 4.74 Billion | |
|
|
|
|
Mercado global de adyuvantes para vacunas, por tipo de producto (adyuvantes particulados, emulsiones adyuvantes, componentes patógenos, adyuvantes combinados, adyuvantes liposomales, adyuvantes de carbohidratos, alumbre y otros), vía de administración (vía intramuscular, vía subcutánea, vía intranasal, vía oral, vía intradérmica y otras), tipo de enfermedad (enfermedades infecciosas, cáncer y otras), aplicación (aplicación de investigación y aplicación comercial), categoría de aplicación (adyuvantes para vacunas humanas y adyuvantes para vacunas veterinarias), usuario final (pediátrico, adultos y otros): tendencias de la industria y pronóstico hasta 2030.
Análisis y tamaño del mercado de adyuvantes para vacunas
Se espera que el mercado mundial de adyuvantes para vacunas experimente un crecimiento significativo en los próximos años. Factores como la creciente prevalencia de enfermedades infecciosas , la expansión de los programas de vacunación y los avances en el desarrollo de adyuvantes están impulsando la expansión del mercado. Además, el creciente enfoque en el desarrollo de vacunas para enfermedades emergentes y la necesidad de mejorar la eficacia y la seguridad de las vacunas contribuyen aún más al crecimiento del mercado.
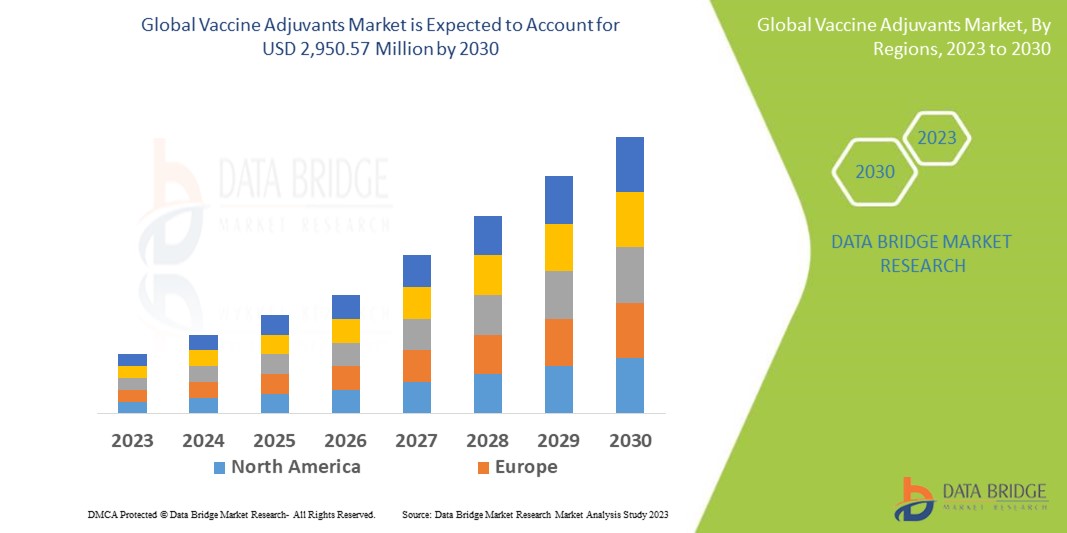
Data Bridge Market Research analiza que el mercado mundial de adyuvantes de vacunas, que fue de USD 1.350,6 millones en 2022, se disparará hasta USD 2.950,57 millones para 2030, y se espera que experimente una CAGR del 13,37% durante el período de pronóstico. Esto indica que el valor de mercado. Se espera que las enfermedades infecciosas dominen el segmento de tipos de enfermedades del mercado de adyuvantes de vacunas debido a la creciente demanda de vacunas para enfermedades infecciosas. Además de los conocimientos sobre escenarios de mercado como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis profundo de expertos, epidemiología de pacientes, análisis de cartera, análisis de precios y marco regulatorio.
Alcance y segmentación del mercado de adyuvantes para vacunas
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2015-2020) |
|
Unidades cuantitativas |
Ingresos en millones de USD, volúmenes en unidades, precios en USD |
|
Segmentos cubiertos |
Tipo de producto (adyuvantes particulados, emulsiones adyuvantes, componentes patógenos, adyuvantes combinados, adyuvantes liposomales, adyuvantes de carbohidratos, alumbre y otros), vía de administración (vía intramuscular, vía subcutánea, vía intranasal, vía oral, vía intradérmica y otras), tipo de enfermedad (enfermedades infecciosas, cáncer y otras), aplicación (aplicación de investigación y aplicación comercial), categoría de aplicación (adyuvantes para vacunas humanas y adyuvantes para vacunas veterinarias), usuario final (pediátrico, adultos y otros) |
|
Países cubiertos |
EE. UU., Canadá, México, Perú, Brasil, Argentina, Resto de Sudamérica, Alemania, Italia, Reino Unido, Francia, España, Países Bajos, Bélgica, Suiza, Turquía, Rusia, Hungría, Lituania, Austria, Irlanda, Noruega, Polonia, Resto de Europa, Japón, China, India, Corea del Sur, Australia, Singapur, Malasia, Tailandia, Indonesia, Filipinas, Vietnam, Resto de Asia Pacífico, Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Kuwait, Israel, Egipto, Resto de Medio Oriente y África |
|
Actores del mercado cubiertos |
Pfizer Inc. (EE. UU.), GlaxoSmithKline plc (Reino Unido), Astrazeneca (Reino Unido), Abbott (EE. UU.), 3M (EE. UU.), Adjuvatis (Francia), Merck KGaA (Alemania), Croda International Plc (Reino Unido), Novavax, Inc. (EE. UU.), InvivoGen (Francia), CSL Limited, SPI Pharma (EE. UU.), Phibro Animal Health Corporation (EE. UU.), SEPPIC (Francia), Agenus Inc. (EE. UU.), OZ Biosciences (Francia), Kineta, Inc. (EE. UU.), vaxine (Australia), Astellas Pharma Inc. (Japón), CureVac AG (Alemania) y Bavarian Nordic (Dinamarca) |
|
Oportunidades de mercado |
|
Definición de mercado
Los adyuvantes de vacunas son compuestos que se consumen para mejorar la respuesta inmunogénica del organismo contra los antígenos. Los adyuvantes de vacunas se utilizan para una amplia gama de aplicaciones.
Dinámica del mercado mundial de adyuvantes para vacunas
Conductores
- Aumento de la carga mundial de enfermedades
La creciente prevalencia de enfermedades infecciosas y la aparición de nuevos patógenos siguen planteando importantes desafíos para la atención sanitaria a nivel mundial. Esto impulsa la demanda de vacunas eficaces y, a su vez, de adyuvantes para vacunas. La necesidad de mejorar la eficacia de las vacunas y la capacidad de brindar protección contra una gama más amplia de patógenos están impulsando el desarrollo y el uso de vacunas adyuvantes.
- Ampliación de los programas de vacunación
Los gobiernos y las organizaciones de atención sanitaria de todo el mundo están ampliando activamente los programas de vacunación para prevenir la propagación de enfermedades infecciosas. Esto incluye la inmunización sistemática, las campañas nacionales de inmunización y las iniciativas de vacunación en respuesta a epidemias o pandemias. La demanda de adyuvantes surge de la necesidad de mejorar la respuesta inmunitaria y mejorar la eficacia de las vacunas utilizadas en estos programas.
- Creciente atención a la atención sanitaria preventiva
Cada vez se hace más hincapié en las medidas de prevención sanitaria, incluida la vacunación, para reducir la carga de enfermedades infecciosas. El reconocimiento de la relación coste-eficacia y los beneficios para la salud pública de las vacunas ha llevado a prestar mayor atención a los programas de inmunización. Los adyuvantes de las vacunas desempeñan un papel crucial en la mejora de la eficacia de las vacunas y en la consecución de la respuesta inmunitaria deseada, lo que favorece el crecimiento del mercado.
- Creciente inversión en centros de salud
Otro factor importante que impulsa el crecimiento del mercado es la creciente atención a la mejora de las condiciones de los centros de atención sanitaria y la mejora de la infraestructura sanitaria en general. El creciente número de asociaciones y colaboraciones estratégicas entre los actores públicos y privados en relación con la financiación y la aplicación de nuevas y mejores tecnologías está creando otras oportunidades de mercado lucrativas.
Oportunidades
- Avances tecnológicos en la fabricación de vacunas
El avance de las tecnologías de fabricación de vacunas, como las tecnologías basadas en cultivos celulares y ADN recombinante, ha permitido la producción de vacunas más complejas y específicas. Los adyuvantes desempeñan un papel fundamental en la optimización de estas formulaciones avanzadas de vacunas y en la mejora de su inmunogenicidad. A medida que evoluciona el panorama de fabricación de vacunas, se espera que aumente la demanda de adyuvantes que puedan complementar y mejorar estas tecnologías.
- Avances en las tecnologías adyuvantes
El campo de la investigación y el desarrollo de adyuvantes avanza continuamente, lo que conduce al descubrimiento y desarrollo de nuevas y mejores tecnologías de adyuvantes. Los adyuvantes novedosos ofrecen perfiles de seguridad mejorados, respuestas inmunitarias mejoradas y mejor compatibilidad con las formulaciones de vacunas. El desarrollo de plataformas de adyuvantes innovadoras impulsa el mercado al permitir la creación de vacunas más efectivas y personalizadas.
Restricciones
- Requisitos reglamentarios estrictos
Los adyuvantes de las vacunas están sujetos a un riguroso escrutinio regulatorio debido a su participación directa en la respuesta inmunitaria y a consideraciones de seguridad. El proceso de aprobación regulatoria de las vacunas adyuvantes puede ser complejo y llevar mucho tiempo. Los estrictos requisitos regulatorios pueden plantear desafíos para las empresas que desarrollan y comercializan adyuvantes de vacunas, lo que genera demoras en la entrada al mercado y mayores costos.
- Preocupaciones de seguridad y percepción pública
Las preocupaciones sobre la seguridad de las vacunas adyuvantes pueden afectar su aceptación en el mercado. Aunque en general se considera que los adyuvantes son seguros, los eventos adversos poco frecuentes o las controversias públicas relacionadas con la seguridad de las vacunas pueden erosionar la confianza del público en las vacunas adyuvantes. Comunicar al público de manera eficaz el perfil de seguridad y los beneficios de las vacunas adyuvantes es crucial para su adopción en el mercado.
Desafíos
- Obstáculos regulatorios y procesos de aprobación
Los adyuvantes de las vacunas están sujetos a estrictos requisitos regulatorios y obtener la aprobación regulatoria puede ser un desafío importante. Los adyuvantes se consideran una parte integral de la formulación de la vacuna y su seguridad, eficacia y estabilidad deben evaluarse exhaustivamente. El proceso regulatorio a menudo implica estudios preclínicos y clínicos extensos, que pueden requerir mucho tiempo y ser costosos. Navegar por el complejo panorama regulatorio y cumplir con los requisitos plantean desafíos para las empresas que desarrollan y comercializan adyuvantes de vacunas.
- Costo y asequibilidad
Los adyuvantes de las vacunas pueden contribuir al coste total del desarrollo y la producción de vacunas. La relación coste-eficacia de los adyuvantes, especialmente cuando se combina con el coste de la producción de vacunas adyuvantes, puede ser un desafío. Equilibrar la necesidad de mejorar la eficacia de las vacunas y la respuesta inmunitaria con las implicaciones de costes es una consideración fundamental tanto para los fabricantes como para los sistemas sanitarios. El desarrollo de adyuvantes rentables y la optimización de los procesos de fabricación para reducir los costes de producción son desafíos constantes en el mercado.
Este informe de mercado de adyuvantes de vacunas proporciona detalles de nuevos desarrollos recientes, regulaciones comerciales, análisis de importación y exportación, análisis de producción, optimización de la cadena de valor, participación de mercado, impacto de los actores del mercado nacional y localizado, analiza oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, análisis estratégico del crecimiento del mercado, tamaño del mercado, crecimientos del mercado de categorías, nichos de aplicación y dominio, aprobaciones de productos, lanzamientos de productos, expansiones geográficas, innovaciones tecnológicas en el mercado. Para obtener más información sobre el mercado de adyuvantes de vacunas, comuníquese con Data Bridge Market Research para obtener un informe de analista, nuestro equipo lo ayudará a tomar una decisión de mercado informada para lograr el crecimiento del mercado.
Acontecimientos recientes
- En abril de 2022, GSK y SK Bioscience presentaron una solicitud de licencia de productos biológicos al Ministerio de Seguridad Alimentaria y Farmacéutica de Corea (KMFDS) para su SKYCovione tras los datos clínicos positivos de la fase III. Se trata de una vacuna candidata contra la COVID-19 basada en proteínas recombinantes con el adyuvante pandémico de GSK
- En febrero de 2022, Sanofi y GSK tenían la intención de presentar datos de sus ensayos de refuerzo y de eficacia de la fase 3 como base para las solicitudes regulatorias para una vacuna contra la COVID-19.
Alcance del mercado mundial de adyuvantes para vacunas
El mercado de adyuvantes para vacunas está segmentado en función del tipo de producto, la vía de administración, el tipo de enfermedad, la aplicación, la categoría de aplicación y el usuario final. El crecimiento entre estos segmentos le ayudará a analizar los segmentos de crecimiento reducidos en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Tipo de producto
- Adyuvantes particulados
- Emulsiones adyuvantes
- Componentes patógenos
- Adyuvantes combinados
- Adyuvantes liposomales
- Adyuvantes de carbohidratos
- Alumbre
- Otros
Vía de administración
- Vía intramuscular
- Vía subcutánea
- Vía intranasal
- Vía oral
- Vía intradérmica
- Otros
Tipo de enfermedad
- Enfermedades infecciosas
- Cáncer
- Otros
Solicitud
- Solicitud de investigación
- Aplicación comercial
Categoría de aplicación
- Adyuvantes de vacunas humanas
- Adyuvantes de vacunas veterinarias
Usuario final
- Pediátrico
- Adultos
- Otros
Análisis y perspectivas regionales del mercado de adyuvantes para vacunas
Se analiza el mercado de adyuvantes de vacunas y se proporcionan información y tendencias del tamaño del mercado por país, tipo de producto, vía de administración, tipo de enfermedad, aplicación, categoría de aplicación y usuario final como se mencionó anteriormente.
The countries covered in the vaccine adjuvants market report are U.S., Canada, Mexico, Peru, Brazil, Argentina, Rest of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia Pacific, South Africa, Saudi Arabia, U.A.E, Kuwait, Israel, Egypt, Rest of the Middle East and Africa.
North America is expected to dominate the vaccine adjuvants market owing to the well-established veterinary healthcare infrastructure.
Asia-Pacific is projected to score the highest growth rate and exhibit the highest CAGR for the forecast period. This is because of the rising expenditure to develop veterinary healthcare infrastructure and the increased focus of the government on immunization programs.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The vaccine adjuvants market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for the vaccine adjuvants market, impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the vaccine adjuvants market. The data is available for the historic period 2015-2020.
Competitive Landscape and Vaccine Adjuvants Market Share Analysis
The vaccine adjuvants market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the vaccine adjuvants market.
Some of the major players operating in the vaccine adjuvants market are:
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- Astrazeneca (U.K.)
- Abbott (U.S.)
- 3M (U.S.)
- Adjuvatis (France)
- Merck KGaA (Germany)
- Croda International Plc (U.K.)
- Novavax, Inc. (U.S.)
- InvivoGen (France)
- CSL Limited (Australia)
- SPI Pharma (Estados Unidos)
- Phibro Animal Health Corporation (Estados Unidos)
- SEPPIC (Francia)
- Agenus Inc. (Estados Unidos)
- OZ Biosciences (Francia)
- Kineta, Inc. (Estados Unidos)
- vacuna (Australia)
- Astellas Pharma Inc. (Japón)
- CureVac AG (Alemania)
- Nórdico bávaro (Dinamarca)
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.