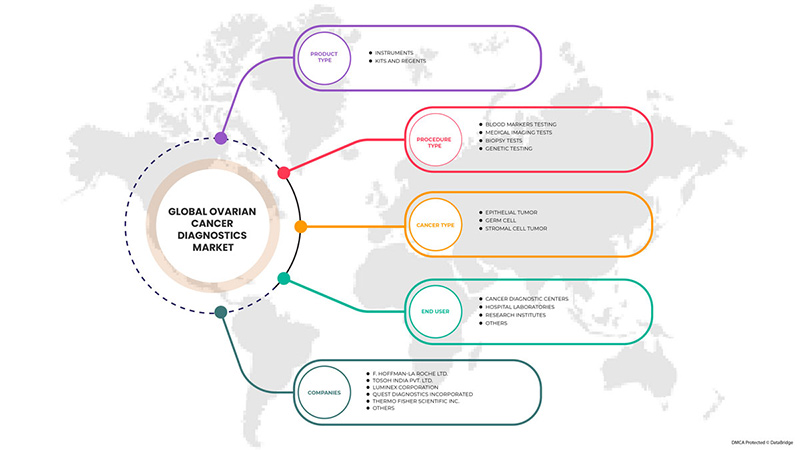
Mercado mundial de diagnóstico de cáncer de ovario, por tipo de producto (instrumentos, kits y reactivos), tipo de procedimiento (prueba de biopsia, prueba de imágenes médicas, pruebas de marcadores sanguíneos y pruebas genéticas ), tipo de cáncer (células germinales, tumores epiteliales y tumores de células del estroma), usuario final (centros de diagnóstico de cáncer, laboratorios hospitalarios, institutos de investigación y otros): tendencias de la industria y pronóstico hasta 2030.
Análisis y perspectivas del mercado de diagnóstico del cáncer de ovario
El cáncer de ovario es el tipo de cáncer que se forma en los tejidos del ovario (una de las dos glándulas reproductoras femeninas en las que se forman los óvulos). La mayoría de los cánceres de ovario son cánceres epiteliales de ovario (cáncer que comienza en las células de la superficie del ovario) o tumores malignos de células germinales (cáncer que comienza en los óvulos). Las pruebas y procedimientos que se utilizan para diagnosticar el cáncer de ovario incluyen el examen pélvico, pruebas de diagnóstico por imágenes, análisis de sangre, cirugía y otros. Durante un examen pélvico, el médico introduce los dedos enguantados en la vagina y, al mismo tiempo, presiona una mano sobre el abdomen para sentir (palpar) los órganos pélvicos.
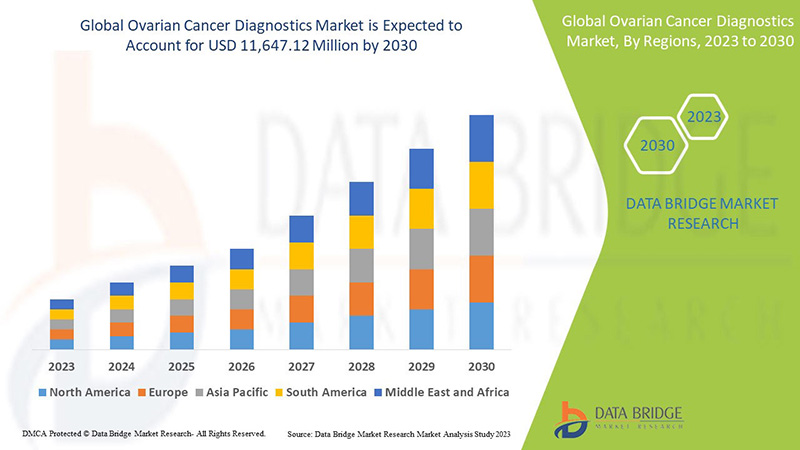
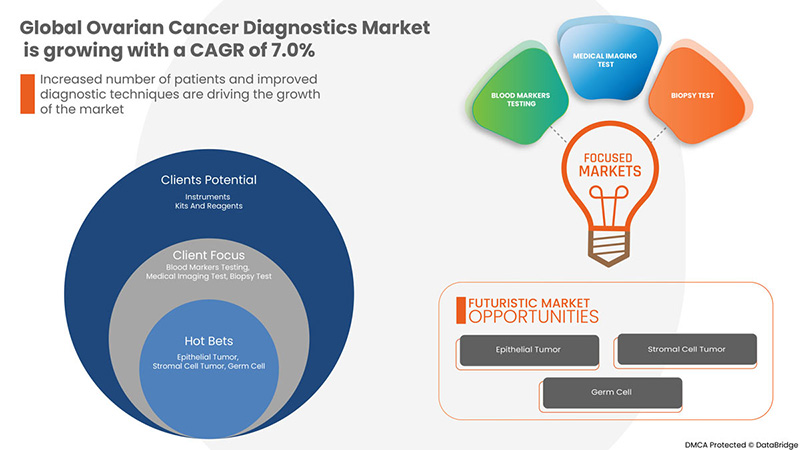
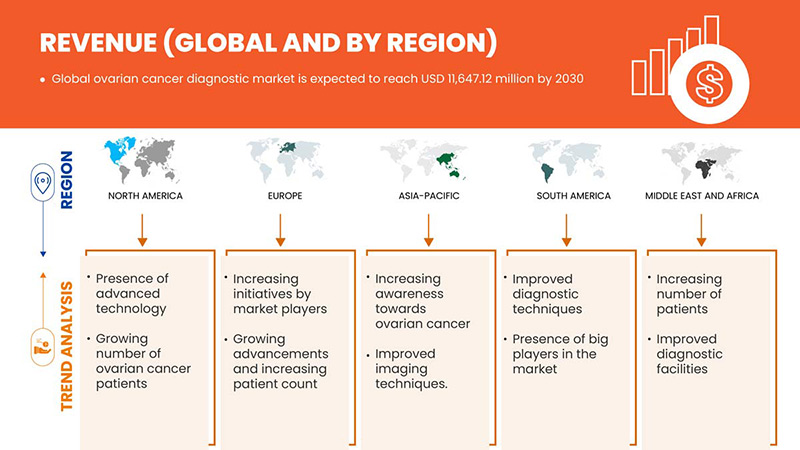
Se espera que el mercado mundial de diagnóstico de cáncer de ovario crezca en el período de pronóstico de 2023 a 2030. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 7,0% en el período de pronóstico de 2023 a 2030 y se espera que alcance los USD 11.647,12 millones para 2030.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2020-2016) |
|
Unidades cuantitativas |
Ingresos en millones de USD |
|
Segmentos cubiertos |
Por tipo de producto (instrumentos, kits y reactivos), tipo de procedimiento (prueba de biopsia, prueba de imágenes médicas, prueba de marcadores sanguíneos y prueba genética), tipo de cáncer (células germinales, tumores epiteliales y tumores de células del estroma), usuario final (centros de diagnóstico de cáncer, laboratorios hospitalarios, institutos de investigación y otros) |
|
Países cubiertos |
Estados Unidos, Canadá y México, Alemania, Francia, Reino Unido, Italia, España, Países Bajos, Rusia, Suiza, Turquía, Bélgica y resto de Europa, China, Japón, India, Corea del Sur, Australia, Singapur, Tailandia, Malasia, Indonesia, Filipinas y resto de Asia-Pacífico, Brasil, Argentina y resto de Sudamérica, Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel y resto de Oriente Medio y África. |
|
Actores del mercado cubiertos |
Español F. Hoffmann-La Roche Ltd., Tosoh India Pvt. Ltd., Luminex Corporation, Quest Diagnostics Incorporated, Thermo Fisher Scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratories, Inc., R&d systems, Inc., Foundation medicine, Inc., Biosupply ltd, Lcm genect srl, Inex innovate private limited, Abcam plc., Monobind Inc., Fujirebio, Mp biomedicals, Biovision Inc., Boster biological technology, Biogenix Inc. Pvt. Ltd., Genway biotech y Lifespan biosciences, Inc. |
Definición del mercado de diagnóstico global del cáncer de ovario
El cáncer de ovario es más común en mujeres de entre 50 y 79 años. Se está volviendo más frecuente a medida que crece la población geriátrica mundial y hay un mayor énfasis en la detección y el tratamiento tempranos, lo que se espera que acelere el desarrollo del mercado de diagnóstico del cáncer de ovario. El aumento de la inversión gubernamental en la concienciación sobre la detección temprana del cáncer, así como el aumento del gasto en atención médica, también impulsarían el crecimiento empresarial. La obesidad parece desempeñar un papel importante en el desarrollo del cáncer de ovario. Otras opciones de estilo de vida que pueden aumentar el riesgo incluyen fumar, beber y no tener hijos. Dado que el cáncer de ovario no es fácilmente detectable, las mujeres que corren el riesgo de desarrollar la enfermedad deben someterse a pruebas de rutina para identificar la enfermedad temprano, lo que permite que el mercado se expanda.
El mercado mundial de diagnóstico de cáncer de ovario está creciendo en el año de pronóstico debido al aumento de los actores del mercado y la disponibilidad de servicios avanzados. Junto con esto, los fabricantes están involucrados en actividades de I+D para lanzar nuevos servicios en el mercado. La creciente investigación en el diagnóstico y desarrollo del cáncer de ovario está impulsando aún más el crecimiento del mercado. Sin embargo, las dificultades en las técnicas de detección del cáncer de ovario podrían obstaculizar el crecimiento del mercado mundial de diagnóstico de cáncer de ovario en el período de pronóstico.
Dinámica del mercado mundial de diagnóstico del cáncer de ovario
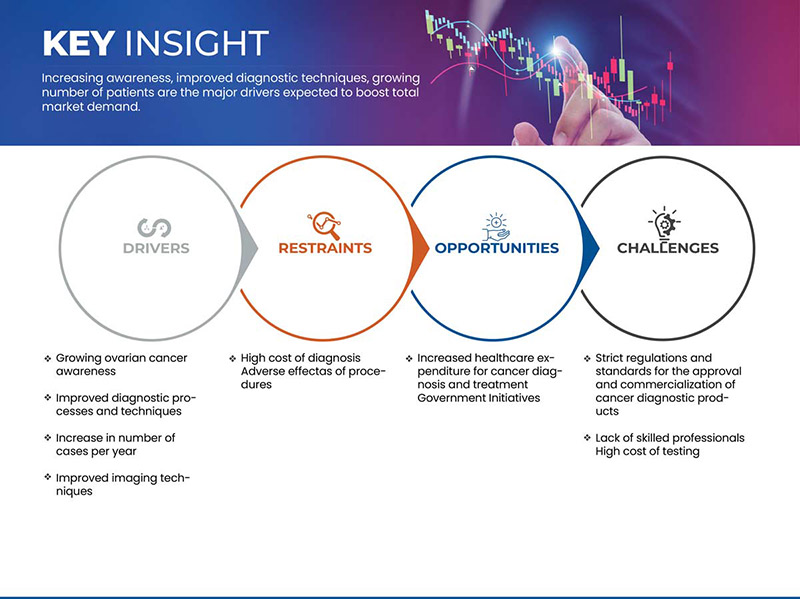
Conductores
- Aumenta la concienciación sobre el cáncer de ovario
La creciente conciencia sobre el cáncer de ovario ha generado una mayor demanda de detección oportuna del cáncer, lo que ha impulsado el crecimiento del mercado.
El cáncer de ovario es una de las principales causas del aumento de las tasas de mortalidad entre las poblaciones femeninas en todo el mundo, lo que impulsará el crecimiento del mercado durante los próximos cinco años. El cáncer de ovario y los quistes son cada vez más comunes debido a diversos factores, como los ambientales y las mutaciones genéticas.
El cáncer de ovario es un tipo de cáncer que afecta a los órganos productores de óvulos de la mujer, los ovarios. El cáncer de ovario es difícil de diagnosticar porque los síntomas son vagos y, a menudo, se detectan solo después de que el cáncer se ha propagado al estómago y la pelvis, lo que dificulta su curación.
Como resultado, se requieren mejores procesos y técnicas de diagnóstico para determinar el estadio del cáncer que se debe tratar. Además, la creciente tasa de mortalidad por cáncer de ovario es preocupante, lo que pone de relieve la importancia de la detección temprana para poder brindar tratamiento.
Debido al aumento de la conciencia sobre el cáncer de ovario, se espera que actúe como un factor impulsor del crecimiento del mercado.
- Procesos y técnicas de diagnóstico mejorados
Las pruebas y exámenes de detección se utilizan para detectar una enfermedad, como el cáncer, en personas que no presentan ningún síntoma. Se han realizado muchas investigaciones para desarrollar una prueba de detección del cáncer de ovario, pero hasta ahora no se ha obtenido mucho éxito. Las dos pruebas que se utilizan con más frecuencia (además de un examen pélvico completo) para detectar el cáncer de ovario son la ecografía transvaginal (TVUS) y el análisis de sangre CA-125.
La ecografía transvaginal transvaginal (TVUS) es una prueba que utiliza ondas sonoras para observar el útero, las trompas de Falopio y los ovarios mediante la introducción de una sonda de ultrasonido en la vagina. Puede ayudar a encontrar una masa (tumor) en el ovario, pero en realidad no puede determinar si una masa es cancerosa o benigna. Cuando se utiliza para la detección, la mayoría de las masas que se encuentran no son cancerosas.
El análisis de sangre CA-125 mide la cantidad de una proteína llamada CA-125 en la sangre. Muchas mujeres con cáncer de ovario tienen niveles altos de CA-125. Este análisis puede ser útil como marcador tumoral para ayudar a orientar el tratamiento en mujeres que se sabe que tienen cáncer de ovario, ya que un nivel alto suele disminuir si el tratamiento está funcionando. Sin embargo, controlar los niveles de CA-125 no es tan útil como una prueba de detección del cáncer de ovario.
Por lo tanto, debido al aumento de procesos y técnicas de diagnóstico mejorados, se espera que actúe como un factor impulsor del crecimiento del mercado.
RESTRICCIONES
Alto costo del diagnóstico
En todo el mundo, los costos del tratamiento del cáncer han aumentado. Las industrias de la salud se enfrentan a varios desafíos, como los costos médicos de la atención oncológica. El costo de la atención oncológica en 2010 fue de USD 124.60 mil millones y se proyecta que aumentará a USD 173.00 mil millones para 2020, siendo los precios de los medicamentos contra el cáncer y la atención hospitalaria aguda los principales impulsores. Por lo tanto, el aumento del costo de la producción de agentes de diagnóstico está obstaculizando el crecimiento del mercado.
Falta de profesionales cualificados
Los profesionales de la salud que participan en el proceso de diagnóstico tienen la obligación y la responsabilidad ética de emplear habilidades de razonamiento clínico, evaluar y tratar los problemas médicos de un paciente. Cuando un diagnóstico es preciso y se realiza de manera oportuna, el paciente tiene la mejor oportunidad de obtener un resultado de salud positivo porque la toma de decisiones clínicas se adaptará a una comprensión correcta del problema de salud del paciente. La falta de profesionales capacitados puede obstaculizar el proceso de recuperación del paciente y, por lo tanto, puede obstaculizar el crecimiento del mercado.
OPORTUNIDADES
Aumento del gasto sanitario en diagnóstico y tratamiento del cáncer
En todo el mundo, las actividades de I+D están aumentando debido al gasto en salud pública con resultados económicos, mientras que la industria de la salud ocupa el segundo lugar entre todas las industrias en lo que respecta a la cantidad gastada en atención médica. El aumento del gasto en atención médica puede dar lugar a una mejor provisión de oportunidades de I+D. Se prevé que aumente la demanda de diagnósticos de cáncer de ovario.
El aumento del gasto sanitario para el tratamiento del cáncer también ayuda a los pacientes a acceder a diagnósticos y tratamientos avanzados sin complicaciones para una recuperación rápida. El gasto en atención sanitaria se compone de una combinación de pagos directos (las personas pagan por su propia atención), gastos gubernamentales y fuentes que incluyen seguros de salud y actividades de organizaciones no gubernamentales (ONG). Debido a este aumento del gasto sanitario para el tratamiento del cáncer, actúa como una oportunidad para el crecimiento del mercado.
DESAFÍOS
Normas y reglamentos estrictos para la aprobación y comercialización de productos de diagnóstico del cáncer
Las estrictas normas para la comercialización de cualquier producto en el mercado están demostrando ser un gran desafío para los fabricantes de productos de diagnóstico del cáncer en los EE. UU. y la región europea. Cada país tiene sus propias normas y cuenta con un organismo diferente para los procedimientos regulatorios.
En los EE. UU., los fabricantes exigen la aprobación de la autorización de comercialización de los productos de diagnóstico in vitro para uso humano. El producto debe estar etiquetado de acuerdo con las normas de etiquetado. Los establecimientos involucrados en la producción y distribución de dispositivos médicos destinados a la distribución comercial en los EE. UU. deben registrarse en la FDA. El registro proporciona a la FDA la ubicación de las instalaciones de fabricación y los importadores de dispositivos médicos. El registro de un establecimiento no es una aprobación del establecimiento o sus dispositivos por parte de la FDA, es decir, no proporciona a la FDA la autorización para comercializar el dispositivo. A menos que esté exento, se requiere una autorización previa a la comercialización antes de que un dispositivo pueda colocarse para su distribución comercial en los EE. UU.
Los requisitos reglamentarios para la aprobación de la comercialización, así como la declaración de conformidad y el tiempo necesario para la revisión reglamentaria pueden variar para distintos productos. La empresa que no consigue la aprobación reglamentaria perjudica a su negocio porque, sin la aprobación de sus productos, los fabricantes no pueden lanzarlos al mercado y, por este motivo, las estrictas normas y regulaciones para la aprobación y comercialización de productos de diagnóstico del cáncer actúan como un factor restrictivo para el crecimiento del mercado.
Acontecimientos recientes
- En noviembre de 2022, Myriad Genetics Inc. anunció la adquisición de Gateway Genomics, LLC. La adquisición fortalece la cartera de productos de salud femenina de Myriad Genetics, ampliando el acceso a pruebas genéticas personalizadas durante la etapa reproductiva de la vida de la mujer y más allá. Con SneakPeek, Myriad ahora atiende a las mujeres en las primeras etapas de su embarazo, brindándoles información genética basada en datos a lo largo de su vida con la prueba prenatal no invasiva Prequel, la prueba de portadores Foresight y la prueba de cáncer hereditario MyRisk con puntuación de riesgo para todas las ascendencias, lo que ayudará a la empresa a aumentar sus ingresos.
- En octubre de 2022, Quest Diagnostics anunció la nueva fase de colaboración con Decode Health. En la fase inicial de colaboración, las dos partes desarrollaron capacidades de secuenciación de ARN (transcriptoma) basadas en la secuenciación de próxima generación, el análisis y la experiencia clínica de ambas partes. La colaboración es importante ya que los datos basados en biomarcadores pueden ayudar a reducir el tiempo y el coste del desarrollo de nuevas pruebas de diagnóstico e identificar nuevos objetivos farmacológicos para diferentes tipos de cánceres (cáncer de mama, próstata y ovario). Esta colaboración ayuda a la empresa a encontrar caminos innovadores en el campo de la I+D y aumenta la presencia global de la empresa.
Alcance del mercado mundial de diagnóstico del cáncer de ovario
Global ovarian cancer diagnostics market is segmented into product type, procedure type, cancer type and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Instruments
- Kits and Reagents
On the basis of product type, the global ovarian cancer diagnostics market is segmented into instruments and kits and reagents.
Procedure Type
- Blood Markers Testing
- Medical Imaging Test
- Biopsy Tests
- Genetic Testing
On the basis of procedure type, the global ovarian Cancer Diagnostics market is segmented into blood markers testing, medical imaging test, biopsy tests and genetic testing
Cancer Type
- Epithelial Tumor
- Germ Cell
- Stromal Cell Tumor
On the basis of cancer type, the global ovarian cancer diagnostics market is segmented into epithelial tumor, germ cell and stromal cell tumor
End User
- Cancer Diagnostic Centers
- Hospital Laboratories
- Research Institutes
- Others
On the basis of end user, the global ovarian cancer diagnostics market is segmented into cancer diagnostic centers, hospital laboratories, research institutes and others
Global Ovarian Cancer Diagnostics Market Regional Analysis/Insights
The global ovarian cancer diagnostics market is analyzed, and market size insights and trends are provided by country, product type, procedure type, cancer type and end user, as referenced above.
The countries covered in this market report are U.S., Canada and Mexico, Germany, France, U.K, Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium and Rest of Europe, China, Japan, India, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines and Rest of Asia-Pacific, Brazil, Argentina and Rest of South America, South Africa, Saudi Arabia, U.A.E, Egypt, Israel and Rest of Middle East and Africa.
North America is dominating the global ovarian cancer diagnostics market in terms of market share and revenue and will continue to flourish its dominance during the forecast period. This is due to the high prevalence and incidence of neurological disorders in the region, and growing R&D investments and the launch of new products are boosting the market
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Ovarian Cancer Diagnostics Market Share Analysis
El panorama competitivo del mercado de diagnóstico de cáncer de ovario proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y la variedad de productos, el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado de diagnóstico de cáncer de ovario.
Algunos de los principales actores que operan en el mercado global de diagnóstico de cáncer de ovario son F. Hoffmann-La Roche Ltd, Tosoh India Pvt. Ltd., Luminex Corporation, Quest Diagnostics Incorporated, Thermo Fisher Scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratories, Inc., R&d systems, Inc., Foundation medicine, Inc., Biosupply ltd, Lcm genect srl, Inex innovate private limited, Abcam plc., Monobind Inc., Fujirebio, Mp biomedicals, Biovision Inc., Boster biological technology, Biogenix Inc. Pvt. Ltd., Genway biotech y Lifespan biosciences, Inc entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 INDUSTRY INSIGHTS
5.1 CONCLUSION
6 REGULATIONS OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING OVARIAN CANCER AWARENESS
7.1.2 IMPROVED DIAGNOSTIC PROCESSES AND TECHNIQUES
7.1.3 INCREASE IN NUMBER OF NEW CASES EVERY YEAR
7.1.4 IMPROVED IMAGING TECHNIQUES
7.2 RESTRAINS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 ADVERSE EFFECTS OF THE TREATMENT
7.3 OPPORTUNITIES
7.3.1 INCREASING HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 GOVERNMENT INITIATIVES TOWARDS CANCER DIAGNOSTICS
7.4 CHALLENGES
7.4.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED PROFESSIONALS
8 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.3 KITS AND REAGENTS
9 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE
9.1 OVERVIEW
9.2 BLOOD MARKERS TESTING
9.3 MEDICAL IMAGING TEST
9.4 BIOPSY TEST
9.5 GENETIC TESTING
10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 EPITHELIAL TUMOR
10.3 GERM CELL
10.4 STROMAL CELL TUMOR
11 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 CANCER DIAGNOSTIC CENTERS
11.3 HOSPITAL LABORATORIES
11.4 RESEARCH INSTITUTES
11.5 OTHERS
12 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY GEOGRAPHY
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S.
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 FRANCE
12.3.3 U.K.
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 TURKEY
12.3.8 BELGIUM
12.3.9 NETHERLANDS
12.3.10 SWITZERLAND
12.3.11 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 JAPAN
12.4.2 CHINA
12.4.3 SOUTH KOREA
12.4.4 INDIA
12.4.5 AUSTRALIA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 MALAYSIA
12.4.9 INDONESIA
12.4.10 PHILIPPINES
12.4.11 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 U.A.E
12.6.4 EGYPT
12.6.5 ISRAEL
12.6.6 REST OF MIDDLE EAST AND AFRICA
13 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
13.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
13.3 COMPANY SHARE ANALYSIS: EUROPE
13.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 F. HOFFMANN-LA ROCHE LTD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 TOSOH INDIA PVT. LTD.
15.2.1 COMPANY SNAPSHOT
15.2.2 COMPANY SHARE ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION (2022)
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 QUEST DIAGNOSTICS INCORPORATED (2022)
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 THERMO FISHER SCIENTIFIC INC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 ABCAM PLC (2022)
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 1.7.4 RECENT DEVELOPMENT
15.8 BIOSUPPLY LTD
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 BIO-RADBIO LABORATORIES
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOVISION INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 BIOGENIX INC. PVT. LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 BOSTER BIOLOGICAL TECHNOLOGY
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 FOUNDATION MEDICINE
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 FUJIREBIO
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 GENWAY BIOTECH
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 INEX INNOVATIVE PRIVATE LIMITED
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 LCM GENETIC SRL
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 LIFESPAN BIOSCIENCES, INC
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 MP BIOMEDICALS
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
15.2 MONOBIND INC.
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.21 MYRIAD GENETICS, INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 REVENUE ANALYSIS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
15.22 NGENEBIO
15.22.1 COMPANY SNAPSHOT
15.22.2 PRODUCT PORTFOLIO
15.22.3 RECENT DEVELOPMENTS
15.23 R&D SYSTEMS, INC.
15.23.1 COMPANY SNAPSHOT
15.23.2 PRODUCT PORTFOLIO
15.23.3 RECENT DEVELOPMENT
15.24 SIEMENS MEDICAL SOLUTIONS
15.24.1 COMPANY SNAPSHOT
15.24.2 REVENUE ANALYSIS
15.24.3 PRODUCT PORTFOLIO
15.24.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tablas
TABLE 1 24-MONTH EPISODE-OF-CARE COSTS FOR EARLY-STAGE AND LATE-STAGE CANCERS BY PAYER (USD BILLION)
TABLE 2 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 GLOBAL INSTRUMENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 GLOBAL KITS AND REAGENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 6 GLOBAL BLOOD MARKERS TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 GLOBAL MEDICAL IMAGING TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 GLOBAL BIOPSY TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 GLOBAL GENETIC TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 11 GLOBAL EPITHELIAL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 GLOBAL GERM CELL IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 GLOBAL STROMAL CELL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 15 GLOBAL CANCER DIAGNOSTIC CENTERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 GLOBAL HOSPITAL LABORATORIES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 GLOBAL RESEARCH INSTITUTES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 GLOBAL OTHERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 27 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 28 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 29 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 30 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 31 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 32 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 33 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 34 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 35 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 36 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 37 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 38 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 40 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 41 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 42 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 44 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 45 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 48 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 49 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 50 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 52 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 53 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 54 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 56 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 57 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 60 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 61 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 62 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 64 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 65 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 66 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 68 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 69 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 70 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 72 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 73 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 74 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 76 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 77 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 78 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 80 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 81 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 82 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 84 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 85 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 86 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 87 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 88 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 89 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 90 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 92 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 93 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 94 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 95 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 97 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 98 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 103 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 104 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 105 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 106 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 107 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 108 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 109 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 110 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 112 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 113 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 115 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 117 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 118 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 119 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 121 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 122 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 123 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 124 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 125 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 126 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 127 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 128 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 129 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 130 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 131 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 132 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 133 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 134 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 135 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 136 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 137 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 138 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 139 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 140 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 141 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 142 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 143 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 144 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 145 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 146 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 147 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 REST OF SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 149 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 150 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 151 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 152 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 153 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 154 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 155 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 156 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 157 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 158 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 159 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 160 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 161 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 162 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 163 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 165 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 166 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 167 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 168 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 169 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 170 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 171 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 172 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 173 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 174 REST OF MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT OVARIAN CANCER IS EXPECTED TO DRIVE THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET IN 2022 & 2030
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET , AND ASIA-PACIFI IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 14 EUROPE IS THE FASTEST-GROWING MARKET FOR GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET MANUFACTURERS IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGE OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
FIGURE 16 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 17 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 18 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 19 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2022
FIGURE 21 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
FIGURE 22 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, CAGR (2023-2030)
FIGURE 23 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 25 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 26 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 27 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 28 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 29 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 30 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 31 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 32 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 33 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY COUNTRY (2022)
FIGURE 34 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY COUNTRY (2023 & 2030)
FIGURE 35 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 36 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY PRODUCTS (2023-2030)
FIGURE 37 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 38 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 39 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 40 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 42 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 43 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 44 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 47 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 48 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 49 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 50 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 51 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 52 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 53 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 54 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 55 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 56 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 57 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 58 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 59 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 60 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 61 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 62 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 63 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 64 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 65 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.