Mercado global de nanotecnología en dispositivos médicos por producto (dispositivos implantables activos, biochips, materiales implantables, textiles médicos y apósitos para heridas, otros), aplicación (aplicaciones terapéuticas, aplicaciones de diagnóstico, aplicaciones de investigación), país (EE. UU., Canadá, México, Alemania, Italia, Reino Unido, Francia, España, Países Bajos, Bélgica, Suiza, Turquía, Rusia, Resto de Europa, Japón, China, India, Corea del Sur, Australia, Singapur, Malasia, Tailandia, Indonesia, Filipinas, Resto de Asia-Pacífico, Brasil, Argentina, Resto de Sudamérica, Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel, Resto de Medio Oriente y África): tendencias de la industria y pronóstico hasta 2029
Análisis y perspectivas del mercado global de nanotecnología en dispositivos médicos
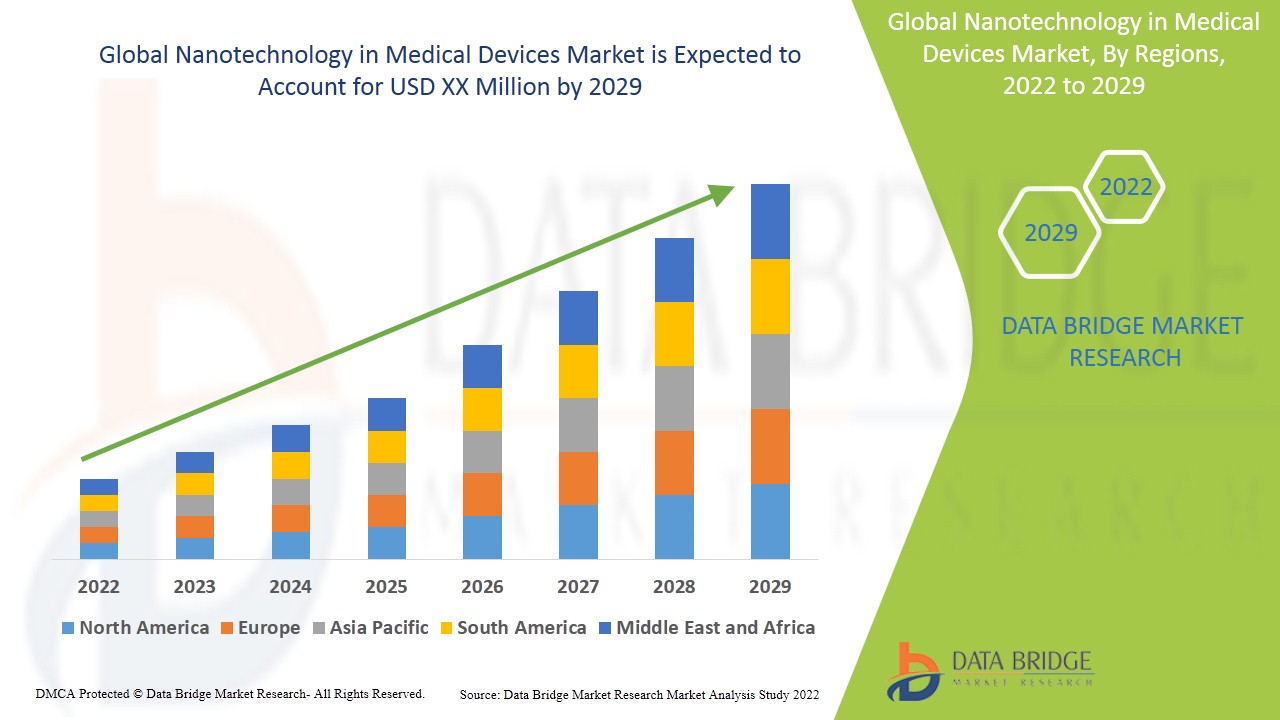
Se espera que el mercado de la nanotecnología en dispositivos médicos experimente un crecimiento del mercado a una tasa del 12,51% en el período de pronóstico de 2022 a 2029. El informe de investigación de mercado de Data Bridge sobre el mercado de la nanotecnología en dispositivos médicos proporciona análisis e información sobre los diversos factores que se espera que prevalezcan durante el período de pronóstico, al tiempo que proporciona sus impactos en el crecimiento del mercado. El aumento del apoyo del gobierno en la provisión de tecnología avanzada está intensificando el crecimiento del mercado de la nanotecnología en dispositivos médicos.
La nanotecnología se refiere al estudio y caracterización de estructuras microminiatura a escala nanométrica. Mejora la selectividad y la sensibilidad y ofrece diagnósticos precisos y rentables, así como una medicación específica.
Los principales factores que se espera que impulsen el crecimiento del mercado de la nanotecnología en dispositivos médicos en el período de pronóstico son el aumento de la población geriátrica. Además, se prevé que la prevalencia de enfermedades en todo el mundo impulse aún más el crecimiento del mercado de la nanotecnología en dispositivos médicos. Además, se prevé que la creciente adopción y demanda de tecnología avanzada y de alto nivel para el tratamiento de enfermedades crónicas impulse aún más el crecimiento del mercado de la nanotecnología en dispositivos médicos. Por otro lado, se prevé que las estrictas normas y regulaciones que consumirán tiempo en la aprobación de productos impidan aún más el crecimiento del mercado de la nanotecnología en dispositivos médicos en el período de tiempo previsto.
Además, el creciente número de solicitudes de los países emergentes ofrecerá más oportunidades potenciales para el crecimiento del mercado de la nanotecnología en dispositivos médicos en los próximos años. Sin embargo, el creciente proceso de desarrollo de dispositivos médicos basados en la nanotecnología podría suponer un mayor desafío para el crecimiento de este mercado en el futuro cercano.
Este informe sobre el mercado de nanotecnología en dispositivos médicos proporciona detalles de los nuevos desarrollos recientes, regulaciones comerciales, análisis de importación y exportación, análisis de producción, optimización de la cadena de valor, participación de mercado, el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, análisis estratégico del crecimiento del mercado, tamaño del mercado, crecimientos del mercado por categorías, nichos de aplicación y dominio, aprobaciones de productos, lanzamientos de productos, expansiones geográficas, innovaciones tecnológicas en el mercado. Para obtener más información sobre el mercado de nanotecnología en dispositivos médicos, comuníquese con Data Bridge Market Research para obtener un informe de analista. Nuestro equipo lo ayudará a tomar una decisión de mercado informada para lograr el crecimiento del mercado.
Alcance y tamaño del mercado mundial de nanotecnología en dispositivos médicos
El mercado de la nanotecnología en dispositivos médicos está segmentado en función del producto y las aplicaciones. El crecimiento entre estos segmentos le ayudará a analizar los segmentos de crecimiento reducido en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para la identificación de las principales aplicaciones del mercado.
- En función del producto, el mercado de la nanotecnología en dispositivos médicos se segmenta en dispositivos implantables activos, biochips , materiales implantables, textiles médicos y apósitos para heridas, entre otros. Los dispositivos implantables activos se han segmentado en dispositivos de control del ritmo cardíaco, dispositivos de ayuda auditiva e implantes de retina. Los biochips se han segmentado además en microarrays de ADN y laboratorio en chip. Los materiales implantables se han segmentado además en materiales de restauración dental y materiales sustitutos óseos.
- Sobre la base de las aplicaciones, el mercado de la nanotecnología en dispositivos médicos se segmenta en aplicaciones terapéuticas, aplicaciones de diagnóstico y aplicaciones de investigación.
Análisis a nivel de país del mercado de nanotecnología en dispositivos médicos
Se analiza el mercado de nanotecnología en dispositivos médicos y se proporcionan información y tendencias del tamaño del mercado por país, producto y aplicaciones como se menciona anteriormente.
Los países cubiertos en el informe del mercado de nanotecnología en dispositivos médicos son EE. UU., Canadá y México en América del Norte, Alemania, Francia, Reino Unido, Países Bajos, Suiza, Bélgica, Rusia, Italia, España, Turquía, Resto de Europa en Europa, China, Japón, India, Corea del Sur, Singapur, Malasia, Australia, Tailandia, Indonesia, Filipinas, Resto de Asia-Pacífico (APAC) en Asia-Pacífico (APAC), Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Resto de Medio Oriente y África (MEA) como parte de Medio Oriente y África (MEA), Brasil, Argentina y Resto de América del Sur como parte de América del Sur.
América del Norte domina el mercado de la nanotecnología en dispositivos médicos debido al desarrollo de la nanotecnología. Además, la creciente presencia de la mayoría de los actores de dispositivos médicos basados en la nanotecnología y las iniciativas adoptadas por el gobierno impulsarán aún más el crecimiento del mercado de la nanotecnología en dispositivos médicos en la región durante el período de pronóstico. Se proyecta que Asia-Pacífico observará una cantidad significativa de crecimiento en el mercado de la nanotecnología en dispositivos médicos debido al aumento de la población geriátrica. Además, se anticipa que la creciente inversión en investigación y desarrollo de la nanotecnología impulsará aún más el crecimiento del mercado de la nanotecnología en dispositivos médicos en la región en los próximos años.
La sección de países del informe de mercado de nanotecnología en dispositivos médicos también proporciona factores de impacto de mercado individuales y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Puntos de datos como volúmenes de consumo, sitios de producción y volúmenes, análisis de importación y exportación, análisis de tendencias de precios, costo de las materias primas, análisis de la cadena de valor ascendente y descendente son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas globales y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales se consideran al proporcionar un análisis de pronóstico de los datos del país.
Crecimiento de la infraestructura de salud Base instalada y penetración de nuevas tecnologías
El mercado de la nanotecnología en dispositivos médicos también le proporciona un análisis detallado del mercado para cada país: el crecimiento del gasto sanitario en equipos de capital, la base instalada de diferentes tipos de productos para el mercado de la nanotecnología en dispositivos médicos, el impacto de la tecnología que utiliza curvas de línea de vida y los cambios en los escenarios regulatorios de la atención médica y su impacto en el mercado de la nanotecnología en dispositivos médicos. Los datos están disponibles para el período histórico 2010-2020.
Análisis del panorama competitivo y la cuota de mercado de la nanotecnología en dispositivos médicos
El panorama competitivo del mercado de la nanotecnología en dispositivos médicos proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y variedad de productos, y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado de la nanotecnología en dispositivos médicos.
Algunos de los principales actores que operan en el mercado de la nanotecnología en dispositivos médicos son 3M, Dentsply Sirona, Thermo Fisher Scientific, PerkinElmer Inc., General Electric Company, Ferro Corporation, Eppendorf AG, Merck KGaA, ZELLMECHANIK DRESDEN, TÜV Rheinland, Medtronic, Boston Scientific Corporation, BIOTRONIK, LivaNova PLC, Demant A/S, Cochlear Ltd., Sonova, MED-EL Medical Electronics y DEKRA, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.