Global Biosimilar Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
64.93 Billion
USD
598.55 Billion
2024
2032
USD
64.93 Billion
USD
598.55 Billion
2024
2032
| 2025 –2032 | |
| USD 64.93 Billion | |
| USD 598.55 Billion | |
|
|
|
|
Segmentación del mercado global de biosimilares, por tipo de producto (escáneres de resonancia magnética, escáneres de tomografía computarizada, escáneres de tomografía por emisión de posición, biosimilar [EEG], dispositivos de electromiografía [EMG], dispositivos de magnetoencefalografía, dispositivos Doppler transcraneal, monitores de presión intracraneal [PIC], electrodos, sensores, geles y cables), clase de fármaco (insulina, hormona de crecimiento humano recombinante [RHGH], factor estimulante de colonias de granulocitos, interferón, eritropoyetina, etanercept, anticuerpos monoclonales, folitropina, glucagón, calcitonina, teriparatida y enoxaparina sódica), tipo de fabricación (fabricación interna y fabricación por contrato), procedimiento (invasivo y no invasivo), enfermedad (accidente cerebrovascular, demencia y epilepsia), indicación (defecto del tabique auricular [CIA], defecto del tabique ventricular [CIV], patente Foramen oval (FOP), estenosis de la válvula aórtica y otros), tipo de terapia (oncología, inmunología, hematología, terapia hormonal, trastornos metabólicos y otros), usuarios finales (hospitales, clínicas, centros de diagnóstico y otros): tendencias de la industria y pronóstico hasta 2032
Tamaño del mercado de biosimilares
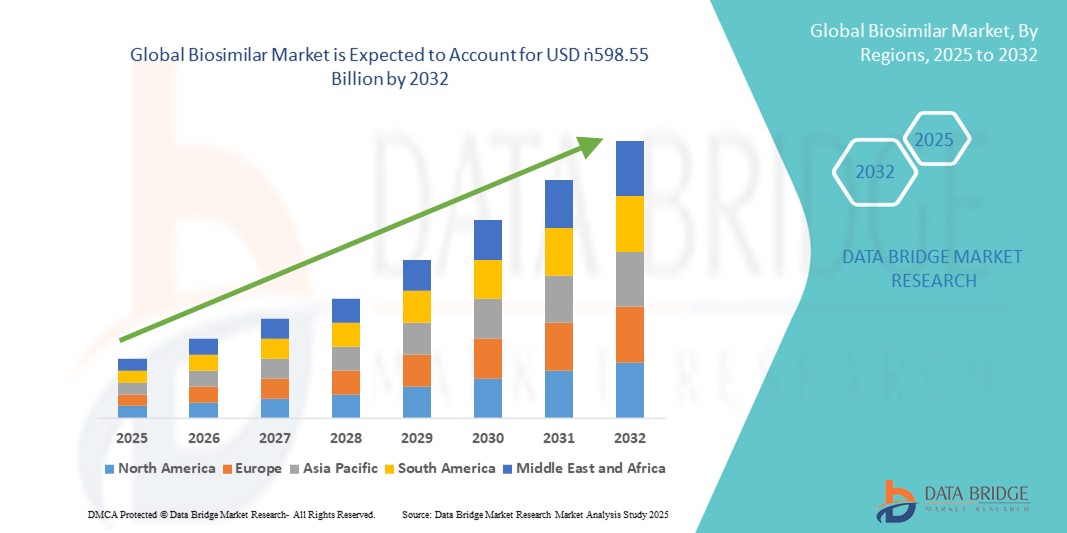
- El tamaño del mercado global de biosimilares se valoró en USD 64,93 mil millones en 2024 y se espera que alcance los USD 598,55 mil millones para 2032 , con una CAGR del 32,00% durante el período de pronóstico.
- La expansión del mercado está impulsada principalmente por el creciente número de vencimientos de patentes de productos biológicos de gran éxito y la creciente prevalencia de enfermedades crónicas como el cáncer, los trastornos autoinmunes y la diabetes, que están impulsando la demanda de alternativas rentables.
- Además, los marcos regulatorios favorables, el aumento de las inversiones de las compañías farmacéuticas y la mayor aceptación por parte de los profesionales sanitarios están acelerando el desarrollo y la adopción de biosimilares en todo el mundo. Estas tendencias contribuyen significativamente a la sólida trayectoria de crecimiento de la industria de los biosimilares.
Análisis del mercado de biosimilares
- Los biosimilares, diseñados como alternativas muy similares y rentables a los medicamentos biológicos aprobados, se están convirtiendo en una parte crucial de los sistemas de atención médica globales, ya que mejoran la accesibilidad al tratamiento, reducen los costos de atención médica y amplían el alcance de los pacientes para enfermedades crónicas y potencialmente mortales como el cáncer, los trastornos autoinmunes y la diabetes.
- La creciente demanda de biosimilares se ve impulsada principalmente por la ola de expiraciones de patentes de productos biológicos de gran éxito, las crecientes presiones sobre el gasto sanitario y una mayor aceptación de los biosimilares entre los proveedores y los pacientes como opciones terapéuticas seguras y eficaces.
- Norteamérica dominó el mercado global de biosimilares con la mayor participación en ingresos, un 42,8 % en 2024, gracias al sólido apoyo regulatorio de la FDA estadounidense, la creciente incorporación de biosimilares a categorías terapéuticas de alto valor y una mayor adopción entre los pagadores que buscan alternativas asequibles a los costosos productos biológicos. Estados Unidos, en particular, ha experimentado una rápida penetración en el mercado gracias a cambios favorables en las políticas, precios competitivos y la presencia de compañías farmacéuticas líderes.
- Se espera que Asia-Pacífico sea la región de más rápido crecimiento en el mercado de biosimilares durante el período de pronóstico debido a la expansión de la infraestructura de atención médica, la creciente incidencia de enfermedades crónicas y las iniciativas gubernamentales de apoyo en países como China, India y Corea del Sur.
- El segmento de oncología dominó el mercado de biosimilares con una participación en los ingresos del 42,2 % en 2024, impulsado por el alto costo de los productos biológicos de referencia, la creciente prevalencia del cáncer a nivel mundial y la rápida adopción de anticuerpos monoclonales biosimilares y terapias de apoyo.
Alcance del informe y segmentación del mercado de biosimilares
|
Atributos |
Perspectivas clave del mercado de biosimilares |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de expertos en profundidad, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de biosimilares
Ampliación de la accesibilidad mediante regulaciones favorables y ahorros de costos
- Una tendencia significativa y en auge en el mercado global de biosimilares es la expansión del apoyo regulatorio y de los marcos de políticas que fomentan aprobaciones más rápidas y una mayor adopción de biosimilares como alternativas rentables a los productos biológicos de alto precio. Esto está transformando la asequibilidad de los tratamientos y la accesibilidad para los pacientes en múltiples áreas terapéuticas.
- Por ejemplo, en 2023, la FDA estadounidense publicó una nueva guía de intercambiabilidad para agilizar la entrada de biosimilares al mercado, mientras que la Agencia Europea de Medicamentos (EMA) mantuvo el liderazgo con el mayor número de aprobaciones de biosimilares a nivel mundial. Estas medidas fortalecen la confianza de la industria e impulsan la entrada competitiva al mercado.
- La creciente presión sobre los costos de la atención médica ha obligado a las aseguradoras y a los gobiernos a priorizar la adopción de biosimilares. En EE. UU., por ejemplo, Amjevita de Amgen, un biosimilar de Humira, se comercializó en 2023 a un costo significativamente menor que el biológico de referencia de AbbVie, lo que sentó un precedente para una mayor adopción. De igual manera, Biocon y Viatris están implementando activamente biosimilares para oncología y diabetes a precios accesibles en Asia-Pacífico.
- Esta tendencia se ve reforzada por la creciente confianza de los médicos, ya que más evidencia práctica confirma que los biosimilares ofrecen una seguridad y eficacia comparables a las de los productos biológicos originales. Las campañas generalizadas de educación para médicos y pacientes están acelerando aún más la adopción en áreas terapéuticas como la oncología, las enfermedades autoinmunes y la diabetes.
- Además, las alianzas entre compañías farmacéuticas globales y actores regionales facilitan un desarrollo y una distribución rentables. Por ejemplo, Samsung Bioepis ha ampliado su alcance mediante colaboraciones con Organon y Biogen para fortalecer su cartera de biosimilares en inmunología y oftalmología.
- Este impulso regulatorio y económico hacia la adopción de biosimilares está transformando fundamentalmente los mercados globales de productos biológicos, con una demanda que aumenta rápidamente tanto en las economías desarrolladas como en las emergentes a medida que las partes interesadas priorizan cada vez más el ahorro de costos y la accesibilidad al tratamiento.
Dinámica del mercado de biosimilares
Conductor
La creciente demanda impulsada por el vencimiento de patentes biológicas y la carga de enfermedades crónicas
- El aumento de la demanda de biosimilares se debe principalmente a la pérdida de la exclusividad de patentes para productos biológicos de gran éxito como Humira, Herceptin y Avastin, lo que crea oportunidades para que los biosimilares de menor costo capturen una participación significativa en el mercado.
- Por ejemplo, en 2023 se lanzaron en EE. UU. varios biosimilares de Humira de empresas como Amgen, Boehringer Ingelheim y Sandoz, lo que generó un entorno de precios competitivos y un acceso más amplio para los pacientes.
- La creciente carga mundial de enfermedades crónicas como el cáncer, los trastornos autoinmunes y la diabetes está intensificando la necesidad de terapias biológicas asequibles, posicionando a los biosimilares como fundamentales para la sostenibilidad del sistema de atención de la salud.
- Los gobiernos y las aseguradoras están implementando cada vez más incentivos de reembolso y políticas de sustitución para fomentar la adopción de biosimilares, creando un entorno favorable para los fabricantes.
- La creciente conciencia entre los médicos y los pacientes sobre la eficacia y la equivalencia de seguridad de los biosimilares en comparación con los originales está impulsando aún más su adopción, respaldada por iniciativas educativas continuas de las autoridades sanitarias.
Restricción/Desafío
Barreras de intercambiabilidad y complejidad de fabricación
- A pesar de las sólidas perspectivas de crecimiento, los desafíos en torno a la intercambiabilidad y la complejidad regulatoria siguen limitando su adopción generalizada. En EE. UU., la designación de intercambiabilidad sigue siendo un obstáculo crítico, ya que no todos los biosimilares tienen derechos de sustitución automática en las farmacias.
- Por ejemplo, si bien Amjevita de Amgen entró en el mercado estadounidense, inicialmente no contaba con la condición de intercambiable, lo que ralentizó la sustitución en comparación con los genéricos tradicionales. Esto sigue siendo un desafío para los nuevos participantes que intentan ganar terreno rápidamente.
- La fabricación de biosimilares también presenta importantes barreras técnicas, ya que los productos biológicos son moléculas grandes y complejas que requieren sistemas avanzados de producción y control de calidad. Cualquier variación en los procesos de producción puede generar inquietudes regulatorias y retrasar las aprobaciones.
- Además, la reticencia de los médicos y la indecisión de los pacientes en ciertas regiones persisten debido a la persistencia de ideas erróneas sobre la eficacia y la seguridad de los biosimilares. La formación continua y los datos reales son esenciales para superar estas percepciones.
- La erosión de precios debido a la feroz competencia plantea otro desafío, ya que las empresas se enfrentan a la presión de reducir costos y gestionar al mismo tiempo los elevados gastos de desarrollo y producción. Esto puede limitar la rentabilidad de las empresas más pequeñas que entran al mercado.
- Superar estos obstáculos a través de vías regulatorias más claras, mejores capacidades de fabricación y una educación más sólida de las partes interesadas será vital para lograr un crecimiento sostenido en el sector de los biosimilares.
Alcance del mercado de biosimilares
El mercado está segmentado según el tipo de producto, clase de fármaco, tipo de fabricación, procedimiento, enfermedad, indicación, tipo de terapia y usuarios finales.
- Por tipo de producto
Según el tipo de producto, el mercado de biosimilares se segmenta en escáneres de resonancia magnética, escáneres de tomografía computarizada, escáneres de tomografía por emisión de positrones, biosimilares (EEG), dispositivos de electromiografía (EMG), dispositivos de magnetoencefalografía, dispositivos Doppler transcraneal, monitores de presión intracraneal (PIC), electrodos, sensores, geles y cables. El segmento de escáneres de resonancia magnética dominó el mercado en 2024 con la mayor participación en los ingresos, impulsado por su papel crítico en oncología y neurología para imágenes de alta resolución y monitoreo de enfermedades. La demanda de MRI se ve impulsada aún más por su amplia aplicación en la detección de tumores, trastornos articulares y afecciones cardiovasculares, en línea con la creciente prevalencia de enfermedades crónicas. Los hospitales y centros de diagnóstico prefieren los biosimilares basados en MRI debido a su confiabilidad, seguridad y mayor precisión diagnóstica. Además, las políticas de reembolso favorables en los mercados desarrollados respaldan una mayor adopción de tecnologías de MRI en entornos clínicos.
Se prevé que el segmento de electrodos registre el mayor crecimiento entre 2025 y 2032 debido a su uso generalizado en diversos procedimientos diagnósticos. Su asequibilidad, su facilidad de uso y la creciente demanda en neurología y cardiología los hacen muy atractivos en mercados emergentes donde la rentabilidad es crucial. La rápida urbanización y el creciente número de pacientes que requieren monitorización de EEG y EMG también están impulsando su adopción. Además, la tendencia de los diagnósticos en el punto de atención y los dispositivos de monitorización portátiles está expandiendo el uso de estos consumibles. El crecimiento del segmento también se ve impulsado por los avances en la ciencia de los materiales, que mejoran la sensibilidad de los electrodos y la comodidad del paciente.
- Por clase de fármaco
Según la clase de fármaco, el mercado de biosimilares se segmenta en insulina, hormona de crecimiento humana recombinante (RHGH), factor estimulante de colonias de granulocitos (G-CSF), interferón, eritropoyetina, etanercept, anticuerpos monoclonales, folitropina, glucagón, calcitonina, teriparatida y enoxaparina sódica. El segmento de anticuerpos monoclonales dominó el mercado en 2024 debido a su importancia crucial en oncología, inmunología y enfermedades autoinmunes. La expiración de patentes de los exitosos anticuerpos monoclonales, junto con sus altos costos de tratamiento, ha creado un fuerte incentivo para el desarrollo de biosimilares. Estos productos biológicos se utilizan ampliamente en el tratamiento del cáncer, la artritis reumatoide y la enfermedad inflamatoria intestinal, lo que los convierte en una opción predilecta para los fabricantes de biosimilares. El segmento se beneficia además de las vías regulatorias que fomentan aprobaciones más rápidas y la aceptación clínica.
Se prevé que el segmento de la insulina experimente el mayor crecimiento entre 2025 y 2032, impulsado por la creciente prevalencia de la diabetes a nivel mundial. El aumento de la población mundial con diabetes, especialmente en Asia-Pacífico, está generando una fuerte demanda de alternativas asequibles a la insulina. Varios biosimilares de insulina han recibido aprobaciones regulatorias, lo que acelera su penetración en el mercado. Los gobiernos y los sistemas de salud están promoviendo activamente los biosimilares de insulina para reducir los costos del tratamiento y mejorar la accesibilidad para los pacientes. Además, las tecnologías de fabricación mejoradas y las alianzas entre compañías farmacéuticas están facilitando una mayor disponibilidad.
- Por tipo de fabricación
Según el tipo de fabricación, el mercado de biosimilares se segmenta en fabricación interna y fabricación por contrato. El segmento de fabricación interna dominó el mercado en 2024, ya que las principales compañías farmacéuticas mantienen el control de la producción para garantizar la calidad y el cumplimiento de las estrictas regulaciones sobre biosimilares. Contar con instalaciones internas permite a las empresas gestionar la propiedad intelectual, controlar la logística de la cadena de suministro y optimizar los costos de producción a largo plazo. También garantiza una calidad constante del producto, fundamental para ganar la confianza de médicos y pacientes en los biosimilares. Las compañías farmacéuticas más grandes con una infraestructura de productos biológicos consolidada siguen dependiendo en gran medida de sistemas internos para mantener su ventaja competitiva.
Se proyecta que el segmento de fabricación por contrato sea el de mayor crecimiento durante el período de pronóstico debido a la creciente tendencia a la externalización entre los desarrolladores de biosimilares medianos y pequeños. Las organizaciones de fabricación por contrato (CMO) ofrecen experiencia especializada, escalabilidad y rentabilidad, lo que facilita la entrada al mercado de nuevos actores. La creciente cartera de biosimilares, combinada con el aumento de la actividad de I+D en los mercados emergentes, está impulsando la demanda de colaboraciones para la externalización. Además, las CMO están ampliando sus capacidades con sistemas avanzados de biorreactores e instalaciones que cumplen con las normativas para satisfacer las necesidades de los clientes globales. Las colaboraciones estratégicas entre compañías farmacéuticas y CMO impulsan aún más la trayectoria de crecimiento de este segmento.
- Por procedimiento
Según el procedimiento, el mercado de biosimilares se segmenta en invasivo y no invasivo. El segmento no invasivo dominó el mercado en 2024 con la mayor participación en los ingresos, debido a la creciente preferencia de los pacientes por opciones terapéuticas menos dolorosas, más seguras y más convenientes. Los enfoques no invasivos se utilizan ampliamente en oncología y el manejo de enfermedades crónicas, donde el cumplimiento terapéutico del paciente es fundamental. Los organismos reguladores y los hospitales también favorecen los tratamientos no invasivos debido a la reducción del riesgo de complicaciones, las estancias hospitalarias más cortas y los menores costos del tratamiento. El auge de la imagenología avanzada, los biosimilares de diagnóstico y las terapias dirigidas consolida aún más el dominio de esta categoría.
Se prevé que el segmento invasivo registre la tasa de crecimiento más rápida entre 2025 y 2032, impulsado por la creciente demanda de intervenciones precisas en enfermedades complejas como los trastornos cardiovasculares y neurológicos. Los biosimilares invasivos son cruciales en cirugías avanzadas y terapias dirigidas donde es necesaria la administración directa de biológicos. El creciente número de pacientes en cuidados críticos que requieren intervenciones quirúrgicas está impulsando su adopción. Además, los avances en las técnicas quirúrgicas mínimamente invasivas están mejorando los perfiles de seguridad, lo que genera una mayor aceptación. El crecimiento del segmento también se ve respaldado por el aumento de las inversiones en infraestructura sanitaria en los países en desarrollo.
- Por enfermedad
Según la enfermedad, el mercado de biosimilares se segmenta en ictus, demencia y epilepsia. El segmento de ictus dominó el mercado en 2024 con la mayor participación en ingresos, principalmente debido a la creciente incidencia mundial de ictus isquémicos y hemorrágicos. Los biosimilares desempeñan un papel fundamental en la mejora de la asequibilidad de las terapias avanzadas utilizadas en la atención post-ictus. Los hospitales y centros de rehabilitación recurren cada vez más a las terapias basadas en biosimilares para gestionar los resultados de la recuperación y reducir la carga financiera de los pacientes. Las iniciativas gubernamentales dirigidas a mejorar el acceso a tratamientos asequibles para el ictus refuerzan aún más el dominio de este segmento.
Se prevé que el segmento de la demencia experimente el mayor crecimiento entre 2025 y 2032, impulsado por el rápido envejecimiento de la población mundial y la creciente prevalencia de la enfermedad de Alzheimer y trastornos relacionados. Ante la presión sobre los sistemas sanitarios para gestionar el aumento de los costes de la atención a la demencia, los biosimilares ofrecen una alternativa rentable para el tratamiento a largo plazo. Las compañías farmacéuticas están desarrollando activamente biosimilares de anticuerpos monoclonales dirigidos a las proteínas beta-amiloide y tau asociadas con la demencia. Además, las herramientas de diagnóstico temprano, combinadas con terapias basadas en biosimilares, están expandiendo su adopción clínica. El crecimiento de este segmento se ve respaldado por iniciativas políticas y financiación de la investigación dirigidas a los trastornos neurodegenerativos.
- Por indicación
Según la indicación, el mercado de biosimilares se segmenta en comunicación interauricular (CIA), comunicación interventricular (CIV), foramen oval permeable (FOP), estenosis valvular aórtica y otros. El segmento de la estenosis valvular aórtica dominó el mercado en 2024 debido a la alta prevalencia de enfermedades cardiovasculares y la necesidad de intervenciones biológicas asequibles. Los biosimilares ayudan a reducir el costo total del reemplazo valvular y las terapias relacionadas, haciendo que los tratamientos sean más accesibles. La creciente adopción tanto en economías desarrolladas como emergentes está reforzando este dominio. Los hospitales también prefieren las intervenciones basadas en biosimilares debido a su eficacia demostrada y al ahorro de costos en grandes poblaciones de pacientes.
Se proyecta que el segmento de comunicación interventricular (CIV) crecerá a su ritmo más rápido entre 2025 y 2032, impulsado por la creciente incidencia de cardiopatías congénitas en bebés y niños. Los biosimilares se incorporan cada vez más a los protocolos de tratamiento debido a su asequibilidad y accesibilidad. Las mejoras en la atención cardíaca pediátrica y el creciente gasto sanitario en las economías emergentes están acelerando su adopción. Además, la creciente colaboración entre los desarrolladores de biosimilares y los hospitales pediátricos está ayudando a ampliar la cartera de tratamientos. Las crecientes campañas de concienciación sobre las cardiopatías congénitas también contribuyen a este crecimiento.
- Por tipo de terapia
Según el tipo de terapia, el mercado de biosimilares se segmenta en oncología, inmunología, hematología, terapia hormonal, trastornos metabólicos y otros. El segmento de oncología dominó el mercado en 2024 con la mayor participación en ingresos, con un 42,2%, gracias al amplio uso de biosimilares de anticuerpos monoclonales en el tratamiento del cáncer. La creciente incidencia mundial del cáncer, junto con los altos costos de los productos biológicos de marca, está impulsando la adopción de biosimilares. Los organismos reguladores han priorizado las vías de aprobación para los biosimilares oncológicos, lo que permite un acceso más rápido para los pacientes. Los hospitales y centros oncológicos están integrando rápidamente los biosimilares en los protocolos de tratamiento para mejorar la asequibilidad y el acceso. La creciente cartera de biosimilares oncológicos refuerza aún más la sólida posición de mercado de este segmento.
Se prevé que el segmento de inmunología registre el mayor crecimiento entre 2025 y 2032, impulsado por la creciente prevalencia de enfermedades autoinmunes como la artritis reumatoide, la psoriasis y la enfermedad inflamatoria intestinal. Los biosimilares ofrecen alternativas rentables a los costosos fármacos biológicos, como los inhibidores del TNF y las terapias dirigidas a la IL. El segmento se ve impulsado aún más por los marcos regulatorios favorables que promueven la sustitución en inmunología. La demanda de los pacientes de opciones de tratamiento asequibles y a largo plazo también impulsa su rápida adopción. Con sólidos datos clínicos que validan su eficacia, los biosimilares de inmunología están ganando una amplia aceptación entre los médicos.
- Por los usuarios finales
En función de los usuarios finales, el mercado de biosimilares se segmenta en hospitales, clínicas, centros de diagnóstico y otros. El segmento hospitalario dominó el mercado en 2024 debido a su papel como centros primarios para la administración de terapias biosimilares en oncología, cardiología y neurología. Los hospitales se benefician del poder adquisitivo a gran escala y de las alianzas establecidas con fabricantes de biosimilares, lo que les permite reducir significativamente los costos de tratamiento. Los programas hospitalarios financiados por el gobierno y las políticas de reembolso de seguros promueven aún más la adopción de biosimilares en estos entornos. Además, los hospitales proporcionan la infraestructura y la experiencia necesarias para el manejo seguro de productos biológicos complejos.
Se proyecta que el segmento de clínicas experimentará el mayor crecimiento entre 2025 y 2032, impulsado por la creciente tendencia de la atención ambulatoria y la expansión de clínicas especializadas para el manejo de enfermedades crónicas. Las clínicas están adoptando cada vez más biosimilares debido a su menor costo y su fácil integración en los protocolos de tratamiento rutinario. Los pacientes prefieren las clínicas por su accesibilidad, tiempos de espera más cortos y un enfoque de atención personalizada. Además, las clínicas desempeñan un papel importante en la expansión del acceso a biosimilares en regiones suburbanas y rurales. Las alianzas entre desarrolladores de biosimilares y redes clínicas impulsan aún más su adopción.
Análisis regional del mercado de biosimilares
- América del Norte dominó el mercado global de biosimilares con la mayor participación en los ingresos del 42,8 % en 2024, liderada por un fuerte apoyo regulatorio de la FDA de EE. UU., la creciente entrada de biosimilares en categorías terapéuticas de alto valor y una mayor adopción entre los pagadores que buscan alternativas asequibles a los costosos productos biológicos.
- El marco regulatorio bien establecido de la región, en particular la vía de aprobación de biosimilares de la FDA, ha fomentado una fuerte entrada al mercado y ha fomentado la innovación entre los fabricantes farmacéuticos.
- El alto gasto en atención médica, la infraestructura sanitaria avanzada y la sólida cobertura de seguros respaldan aún más la rápida adopción de biosimilares como alternativas asequibles a los productos biológicos de marca.
Perspectiva del mercado estadounidense de biosimilares
El mercado estadounidense de biosimilares captó la mayor participación en los ingresos, con un 83%, en 2024 en Norteamérica, impulsado por la fuerte demanda de alternativas biológicas rentables y la rápida expansión de las aplicaciones terapéuticas en oncología e inmunología. Médicos y pacientes adoptan cada vez más los biosimilares debido a su seguridad y eficacia comprobadas, además de un ahorro significativo en comparación con los productos biológicos de marca. El apoyo regulatorio favorable de la FDA y la creciente aceptación por parte de las aseguradoras están acelerando su penetración en hospitales y clínicas especializadas. Además, la entrada de importantes compañías farmacéuticas y la ampliación de la cobertura de reembolso contribuyen significativamente al crecimiento del mercado.
Perspectivas del mercado de biosimilares en Europa
Se proyecta que el mercado europeo de biosimilares se expandirá a una tasa de crecimiento anual compuesta (TCAC) sustancial durante el período de pronóstico, impulsado por vías regulatorias consolidadas y la adopción temprana de las principales clases terapéuticas. Países como Alemania, Francia y el Reino Unido lideran el uso de biosimilares gracias a las sólidas iniciativas gubernamentales para reducir el gasto sanitario. La creciente prevalencia de enfermedades crónicas, sumada a la presión por tratamientos asequibles, está impulsando su adopción en oncología, endocrinología y reumatología. La creciente confianza de los médicos y las estrategias de precios competitivos también están impulsando la penetración de los biosimilares en los canales hospitalarios y de farmacia.
Perspectivas del mercado de biosimilares del Reino Unido
Se prevé que el mercado británico de biosimilares crezca a una CAGR notable durante el período de pronóstico, gracias a las políticas favorables del NHS que promueven activamente la adopción de biosimilares. Los profesionales sanitarios están optando cada vez más por los biosimilares en oncología, inmunología y trastornos metabólicos debido a la presión de los costes y a la necesidad de un mayor acceso para los pacientes. Se espera que la creciente aceptación entre prescriptores y pacientes, junto con licitaciones competitivas y reducciones de precios, acelere la penetración en el mercado. Además, la colaboración entre el NHS y los fabricantes de biosimilares está fomentando la confianza e impulsando una mayor adopción.
Perspectiva del mercado de biosimilares en Alemania
Se espera que el mercado alemán de biosimilares se expanda a una tasa de crecimiento anual compuesta (TCAC) considerable durante el período de pronóstico, impulsado por la posición del país como uno de los primeros en adoptar biosimilares en Europa. Las sólidas políticas sanitarias que fomentan la sustitución, junto con precios competitivos, han convertido a Alemania en un líder en la penetración de biosimilares en todas las áreas terapéuticas. El énfasis en la reducción de los costos sanitarios y la mejora del acceso a los productos biológicos impulsa su rápida adopción en oncología y enfermedades autoinmunes. Además, la innovación local, los programas de concienciación para médicos y los marcos de reembolso estructurados están impulsando el crecimiento del mercado.
Perspectiva del mercado de biosimilares de Asia y el Pacífico
Se prevé que el mercado de biosimilares de Asia-Pacífico crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 25 %, durante el período previsto de 2025 a 2032, impulsado por la creciente carga de enfermedades crónicas, la expansión de la infraestructura sanitaria y la mayor asequibilidad de los biosimilares en países como China, Japón e India. Las iniciativas gubernamentales favorables, junto con la capacidad de fabricación local, están haciendo que los biosimilares sean más accesibles a una población más amplia de pacientes. Además, las colaboraciones estratégicas entre actores nacionales e internacionales están fomentando la innovación y garantizando las aprobaciones regulatorias. La creciente población de clase media de la región y las crecientes inversiones en salud están acelerando aún más la adopción de biosimilares.
Perspectiva del mercado de biosimilares en Japón
El mercado japonés de biosimilares está cobrando impulso gracias al sólido apoyo gubernamental, las necesidades derivadas del envejecimiento de la población y la creciente prevalencia del cáncer y las enfermedades autoinmunes. Los organismos reguladores japoneses han simplificado los procesos de aprobación, lo que ha impulsado la disponibilidad de biosimilares en todas las áreas terapéuticas. La creciente adopción en hospitales y clínicas especializadas, sumada a la creciente confianza de los médicos, está impulsando la demanda. Además, la avanzada infraestructura sanitaria del país y el énfasis en la contención de costes están impulsando una mayor aceptación en el mercado. Las alianzas entre empresas biofarmacéuticas japonesas e internacionales impulsan aún más la expansión del mercado.
Perspectiva del mercado de biosimilares en India
El mercado indio de biosimilares representó la mayor cuota de mercado en ingresos en Asia Pacífico en 2024, gracias a un sólido ecosistema de fabricación nacional y una creciente demanda de productos biológicos asequibles. India se ha posicionado como un centro global para el desarrollo y la producción de biosimilares, abasteciendo tanto al mercado nacional como al internacional. El aumento de casos de diabetes, cáncer y enfermedades autoinmunes está impulsando la adopción en todas las áreas terapéuticas. Además, las iniciativas gubernamentales para mejorar la accesibilidad a la atención médica, junto con la presencia de importantes actores locales, están impulsando una sólida penetración de los biosimilares. Las estrategias de precios competitivos y la creciente confianza de los médicos impulsan aún más el crecimiento del mercado.
Cuota de mercado de biosimilares
La industria de los biosimilares está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Novartis AG (Suiza)
- Orion Pharma AB (Suecia)
- Pfizer Inc. (EE. UU.)
- Samsung Bioepis. (Corea del Sur)
- Coherus BioSciences, Inc. (EE. UU.)
- Amgen Inc. (EE. UU.)
- Lilly USA, LLC (EE. UU.)
- Takeda Pharmaceutical Company Limited. (Japón)
- Bristol-Myers Squibb Company (EE. UU.)
- Merck KGaA (Alemania)
- Teva Pharmaceutical Industries Ltd. (EE. UU.)
- Biocon (India)
- Bayer AG (Alemania)
- AbbVie Inc. (EE. UU.)
- Laboratorios Dr. Reddy Ltd. (India)
- Boehringer Ingelheim International GmbH (Alemania)
- Biogen (EE. UU.)
¿Cuáles son los desarrollos recientes en el mercado global de biosimilares?
- En mayo de 2025, la FDA aprobó Starjemza (ustekinumab-hmny) como el octavo biosimilar de Stelara (ustekinumab), ofreciendo a los pacientes mejores opciones de tratamiento para afecciones reumáticas y gastrointestinales. Esta aprobación destaca la continua expansión de la clase de biosimilares de ustekinumab y promueve una mayor accesibilidad a estas terapias.
- En febrero de 2025, la FDA designó a Selarsdi, un biosimilar de Stelara (ustekinumab), como intercambiable. Esto significa que los farmacéuticos pueden sustituirlo por Stelara sin la intervención del médico una vez transcurridos los períodos de exclusividad, lo que agiliza significativamente el acceso y la adopción por parte de los pacientes.
- En febrero de 2025, la FDA aprobó Merilog (insulina-aspart-szjj), el primer biosimilar de insulina de acción rápida de Novolog (insulina aspart), disponible en formato de pluma precargada y vial. Esto marca un hito en la expansión de opciones de insulina asequibles para pacientes con diabetes.
- En diciembre de 2024, la FDA otorgó la aprobación a Steqeyma (ustekinumab-stba) como el séptimo biosimilar de Stelara (ustekinumab), lo que permite una mayor competencia y amplía las opciones de tratamiento en el cuidado de enfermedades autoinmunes e inflamatorias.
- En octubre de 2024, Accord BioPharma, Inc. anunció que la FDA aprobó Imuldosa (ustekinumab-srlf), un biosimilar de Stelara (ustekinumab), para todas las mismas indicaciones inflamatorias crónicas, incluidas la psoriasis, la artritis psoriásica, la enfermedad de Crohn y la colitis ulcerosa.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.