Europe Electronic Clinical Outcome Assessment Ecoa For Content Licensed Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
704.70 Million
USD
2,067.34 Million
2025
2033
USD
704.70 Million
USD
2,067.34 Million
2025
2033
| 2026 –2033 | |
| USD 704.70 Million | |
| USD 2,067.34 Million | |
|
|
|
|
Segmentación del mercado europeo de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia, por producto (soluciones locales, soluciones en la nube y soluciones web), enfoque (evaluación de resultados informada por el médico [ClinRO], evaluación de resultados informada por el paciente [PRO], evaluación de resultados informada por el observador [ObsRO] y evaluación de resultados de rendimiento [PerfO]), usuario final (proveedores de servicios comerciales, hospitales y centros de trasplantes, laboratorios de investigación e instituciones académicas), plataforma (organizaciones de investigación por contrato, empresas farmacéuticas y biofarmacéuticas , fabricantes de dispositivos médicos , hospitales y laboratorios clínicos, empresas de servicios de consultoría, investigación y academia, entre otros): tendencias del sector y pronóstico hasta 2033.
Tamaño del mercado de la evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia en Europa
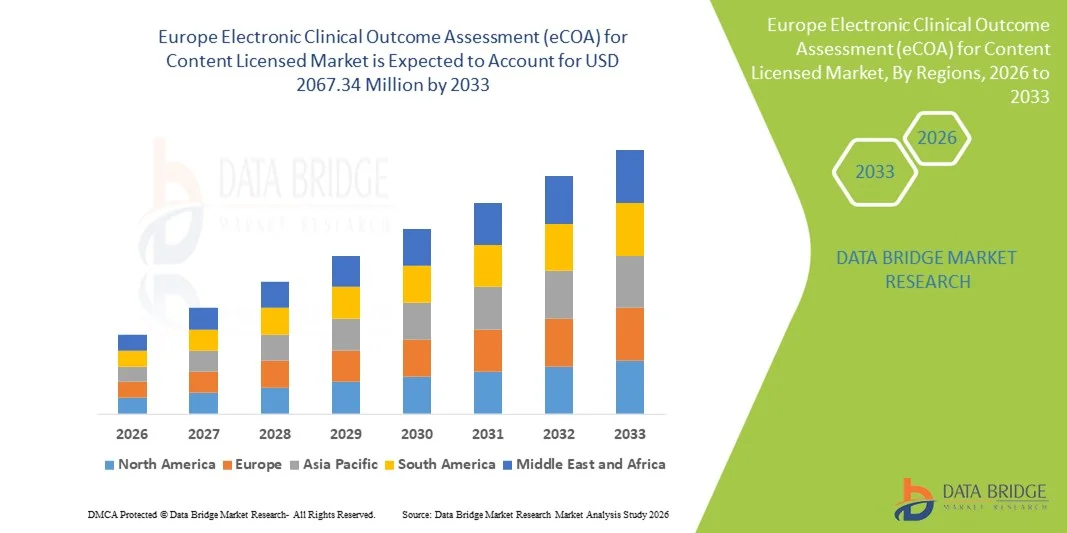
- El tamaño del mercado europeo de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia se valoró en USD 704,7 millones en 2025 y se espera que alcance los USD 2067,34 millones para 2033 , con una CAGR del 14,40 % durante el período de pronóstico.
- El crecimiento del mercado se debe en gran medida a la creciente adopción de tecnologías digitales en ensayos clínicos, el creciente énfasis en la investigación centrada en el paciente y la necesidad de recopilar datos precisos y en tiempo real en todas las áreas terapéuticas. La integración de plataformas en la nube, aplicaciones móviles y resultados electrónicos informados por el paciente (ePRO) impulsa aún más la expansión del mercado de la Evaluación Electrónica de Resultados Clínicos (eCOA) para Contenidos Licenciados.
- Además, la creciente demanda de soluciones de ensayos clínicos que cumplan con las normativas, sean fáciles de usar y escalables está consolidando los sistemas eCOA como un componente fundamental de la investigación clínica moderna. La mayor eficiencia, la reducción de la carga administrativa y la mayor participación del paciente están acelerando la adopción de la Evaluación Electrónica de Resultados Clínicos (eCOA) para soluciones con licencia de contenido, impulsando así significativamente el crecimiento del mercado.
Evaluación electrónica de resultados clínicos (eCOA) para el análisis del mercado de contenido con licencia en Europa
- El crecimiento del mercado de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia se ve impulsado en gran medida por la creciente adopción de resultados electrónicos informados por el paciente (ePRO) y soluciones eCOA en ensayos clínicos, impulsadas por la necesidad de una recopilación de datos precisa y en tiempo real y una mejor monitorización de los pacientes en la investigación farmacéutica y de atención médica.
- La creciente demanda de procesos de ensayos clínicos optimizados, cumplimiento normativo y gestión eficiente de datos está acelerando la adopción de la evaluación electrónica de resultados clínicos (eCOA) para soluciones con licencia de contenido, lo que impulsa significativamente el crecimiento de la industria.
- El Reino Unido dominó el mercado de la Evaluación Electrónica de Resultados Clínicos (eCOA) para Contenido con Licencia, con la mayor cuota de ingresos, de aproximadamente el 38,7 % en 2025, gracias a una infraestructura sanitaria consolidada, una sólida adopción de tecnologías digitales para ensayos clínicos y un sólido respaldo regulatorio para el seguimiento electrónico de los resultados de los pacientes. El Reino Unido está experimentando un crecimiento sustancial en la implementación de eCOA en ensayos clínicos, impulsado por innovaciones en plataformas en la nube, la recopilación de datos móviles y la integración con sistemas centralizados de gestión de ensayos clínicos.
- Se prevé que Alemania sea la región con mayor crecimiento en el mercado de la Evaluación Electrónica de Resultados Clínicos (eCOA) para Contenido con Licencia durante el período de pronóstico, con una sólida tasa de crecimiento anual compuesta (TCAC) de dos dígitos. Este crecimiento se ve impulsado por el aumento de la actividad de ensayos clínicos, el incremento del gasto sanitario, la expansión del acceso a soluciones de salud digital y la creciente adopción de tecnologías de captura electrónica de datos y resultados informados por los pacientes en hospitales, centros de investigación y organizaciones farmacéuticas.
- El segmento de soluciones basadas en la nube dominó con una participación en los ingresos del 45,3 % en 2025, impulsado por la implementación escalable, la facilidad de acceso remoto y la integración con múltiples dispositivos.
Alcance del informe y evaluación electrónica de resultados clínicos (eCOA) para la segmentación del mercado de contenido con licencia
|
Atributos |
Evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia: información clave del mercado |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Europa
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis en profundidad de expertos, epidemiología de pacientes, análisis de la cartera de productos, análisis de precios y marco regulatorio. |
Tendencias del mercado europeo de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia
Creciente adopción de soluciones digitales para ensayos clínicos
- Una tendencia importante en el mercado global de eCOA para contenido con licencia es la creciente adopción de soluciones de ensayos clínicos digitales y remotos, impulsada por la necesidad de una recopilación de datos eficiente, en tiempo real y centrada en el paciente.
- Por ejemplo, en marzo de 2023, CRF Health (ahora parte de Signant Health) expandió su plataforma eCOA en toda Europa para respaldar ensayos clínicos completamente descentralizados, lo que permitió la recopilación electrónica de resultados informados por los pacientes (ePRO) en varios idiomas y protocolos de estudio.
- Las herramientas eCOA digitales reducen errores, aceleran la recopilación de datos y mejoran el cumplimiento del paciente en comparación con los métodos tradicionales basados en papel.
- La pandemia de COVID-19 aceleró esta tendencia, destacando la necesidad de monitoreo remoto de pacientes y captura de datos sin contacto, que se espera que persista después de la pandemia.
- Las empresas farmacéuticas y las organizaciones de investigación por contrato (CRO) están integrando cada vez más las plataformas eCOA con otras tecnologías de ensayos clínicos digitales, como evaluaciones electrónicas de resultados clínicos para biomarcadores digitales y dispositivos portátiles.
Dinámica del mercado de la evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia en Europa
Conductor
Mayor enfoque en los ensayos clínicos centrados en el paciente
- El creciente énfasis en la centralidad del paciente en los ensayos clínicos está impulsando la adopción de plataformas eCOA, que permiten la captura en tiempo real de los resultados informados por el paciente (PRO), el seguimiento de los síntomas y los datos de calidad de vida relacionados con la salud.
- Por ejemplo, en julio de 2024, Parexel International lanzó una nueva solución eCOA diseñada para ensayos oncológicos en toda Europa, que permite a los pacientes informar los resultados directamente desde casa mediante interfaces web o móviles, lo que mejora la participación y la retención.
- Los organismos reguladores, incluida la Agencia Europea de Medicamentos (EMA), recomiendan cada vez más la recopilación digital de PRO para las solicitudes de aprobación de medicamentos, lo que fomenta una adopción más amplia.
- La capacidad de capturar datos longitudinales, estandarizados y de alta calidad de forma remota permite a los patrocinadores optimizar el diseño de los ensayos y reducir la carga del sitio.
- La integración con sistemas de captura electrónica de datos (EDC) y plataformas de gestión de ensayos centralizados proporciona un flujo de trabajo continuo para los patrocinadores de ensayos clínicos y las CRO, lo que fortalece aún más la adopción.
Restricción/Desafío
Privacidad de datos, cumplimiento normativo y costos de implementación
- El mercado de eCOA enfrenta desafíos relacionados con la privacidad de los datos, el cumplimiento del RGPD y los altos costos de implementación , lo que puede retrasar la adopción en todo el mundo.
- Por ejemplo, en septiembre de 2022, varias CRO europeas informaron retrasos en la implementación de soluciones eCOA en Alemania y Francia debido a las estrictas regulaciones de protección de datos y la necesidad de un almacenamiento seguro de datos de los pacientes.
- Garantizar el cumplimiento de las leyes locales, las pautas regulatorias y los requisitos de validación agrega complejidad a la implementación del eCOA, en particular para ensayos multinacionales.
- Además, los costos de integración con los sistemas de ensayos clínicos existentes , incluida la capacitación del personal y la validación del software, pueden ser significativos, lo que plantea barreras para las CRO o los patrocinadores de tamaño pequeño a mediano.
- Para abordar estos desafíos se requiere inversión en ciberseguridad sólida, alineación regulatoria y estrategias de implementación escalables y rentables para respaldar el crecimiento del mercado a largo plazo.
Evaluación electrónica de resultados clínicos (eCOA) para el mercado de contenido con licencia en Europa
El mercado está segmentado en función del producto, el enfoque, el usuario final y la plataforma.
- Por producto
En función del producto, el mercado de la Evaluación Electrónica de Resultados Clínicos (eCOA) para Contenido con Licencia se segmenta en soluciones locales, soluciones en la nube y soluciones web. El segmento de soluciones en la nube dominó el mercado con una participación en los ingresos del 45,3 % en 2025, impulsado por la implementación escalable, la facilidad de acceso remoto y la integración con múltiples dispositivos. Las plataformas en la nube permiten a los patrocinadores, las CRO y los centros clínicos gestionar los datos de forma centralizada, mejorar el cumplimiento normativo y reducir los gastos generales de TI. La alta adopción en ensayos clínicos multicéntricos, los sólidos protocolos de seguridad y los informes en tiempo real refuerzan este dominio. La flexibilidad de los modelos basados en suscripción, la alineación regulatoria y la integración con los sistemas EDC y ePRO impulsan aún más el crecimiento. Las grandes compañías farmacéuticas prefieren las soluciones en la nube para ensayos globales. La pandemia de COVID-19 aceleró la adopción debido a las necesidades de monitorización remota. La mejora del tiempo de actividad y el soporte de los proveedores fortalecen la cuota de mercado. La validación estandarizada en todos los centros garantiza la fiabilidad. La rentabilidad y la optimización del flujo de trabajo impulsan aún más el liderazgo. Las funciones mejoradas de análisis y paneles de control atraen a los usuarios finales. La integración con aplicaciones móviles mejora el cumplimiento del tratamiento por parte del paciente. En general, estos factores sustentan el dominio.
Se espera que el segmento de soluciones locales experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 14,8 %, entre 2026 y 2033, impulsada por organizaciones que requieren control y personalización de datos internos. Hospitales, organizaciones de investigación por contrato (CRO) y empresas de biotecnología con una gobernanza de datos estricta adoptan sistemas locales. La integración con la infraestructura de TI heredada aumenta el atractivo. Los requisitos de alta seguridad para datos clínicos confidenciales promueven la adopción. La creciente demanda en regiones con estrictas regulaciones de privacidad de datos impulsa el crecimiento. Los informes y análisis avanzados mejoran la eficiencia operativa. Las implementaciones a escala empresarial aceleran el uso. La disponibilidad de módulos personalizables atrae a grandes patrocinadores. El soporte de TI interno garantiza un mantenimiento robusto. La creciente necesidad de opciones de implementación híbrida complementa la expansión. La inversión farmacéutica en ensayos de medicina personalizada impulsa aún más la adopción. La capacitación y el soporte técnico fortalecen la implementación. La alineación regulatoria para el cumplimiento de la FDA y la EMA garantiza una adopción acelerada.
- Por enfoque
Según el enfoque, el mercado se segmenta en ClinRO, PRO, ObsRO y PerfO. El segmento de Resultados Reportados por el Paciente (PRO) dominó con una participación en los ingresos del 42,1% en 2025, impulsado por un mayor uso en presentaciones regulatorias, ensayos centrados en el paciente y estudios de evidencia del mundo real. PRO permite la medición directa del estado de salud del paciente, la satisfacción con el tratamiento y las métricas de calidad de vida. La integración con aplicaciones móviles y plataformas en la nube mejora la adopción. El sólido apoyo a las directrices de la FDA y la EMA refuerza la demanda. La alta prevalencia de enfermedades crónicas y ensayos oncológicos impulsa el uso. Los criterios de valoración clínicos dependen cada vez más de los datos de PRO. La compatibilidad con varios idiomas amplía la implementación global. Los recordatorios automatizados mejoran el cumplimiento. La recopilación frecuente de datos aumenta el volumen y la utilidad de los datos. La integración con eCOA, eDiary y dispositivos portátiles fortalece el flujo de trabajo. La inversión farmacéutica en criterios de valoración basados en PRO acelera la adopción. La escalabilidad entre centros y ensayos respalda el dominio. La analítica avanzada permite la segmentación de pacientes y el seguimiento de resultados.
Se espera que el segmento ObsRO crezca a la CAGR más rápida del 15,6% entre 2026 y 2033, debido a la creciente adopción en enfermedades raras, pediatría y resultados informados por los cuidadores. Los datos informados por el observador mejoran la medición en poblaciones que no pueden autoinformarse. El crecimiento de los estudios pediátricos y geriátricos impulsa la demanda. La integración con plataformas de monitorización remota facilita la adopción. La inversión farmacéutica y biotecnológica en ensayos de enfermedades raras apoya la expansión. La estandarización multisitio mejora la fiabilidad de los datos. Las soluciones en la nube y web aceleran la adopción. Los informes de observadores habilitados para dispositivos móviles mejoran el cumplimiento. Los mercados emergentes adoptan cada vez más los enfoques de ObsRO. La aceptación regulatoria de los puntos finales de ObsRO impulsa el crecimiento. La formación y el soporte del sitio fomentan la implementación. Los avances tecnológicos reducen los errores de entrada manual. El alto valor para los ensayos de múltiples partes interesadas acelera el crecimiento.
- Por el usuario final
En función del usuario final, el mercado se segmenta en proveedores de servicios comerciales, hospitales y centros de trasplantes, laboratorios de investigación e instituciones académicas. El segmento de hospitales y centros de trasplantes dominó con una participación en los ingresos del 47,2 % en 2025, debido a la extensa actividad de ensayos clínicos, las grandes poblaciones de pacientes y la necesidad de sistemas integrados de evaluación de resultados. Los hospitales implementan eCOA para el manejo de enfermedades crónicas, ensayos oncológicos y seguimiento de resultados quirúrgicos. La alta adopción en ensayos multicéntricos fortalece el uso. La integración con sistemas EHR y EMR mejora la eficiencia del flujo de trabajo. Los mandatos regulatorios para la recopilación de datos de resultados clínicos impulsan la demanda. Los programas de capacitación y soporte a gran escala garantizan la adopción. La seguridad de los datos y el cumplimiento de las normas HIPAA/GDPR refuerzan la confianza. El soporte multidispositivo mejora la participación del paciente y el médico. Los análisis en tiempo real respaldan las decisiones operativas. Las asociaciones con CRO aumentan la implementación. La monitorización remota de pacientes amplía el alcance. La inversión en investigación clínica hospitalaria impulsa el liderazgo. Los programas de mejora continua mantienen el dominio.
Se espera que el segmento de Proveedores de Servicios Comerciales crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 16,3 %, entre 2026 y 2033, impulsada por las tendencias de externalización en ensayos clínicos y la creciente necesidad de una gestión especializada de eCOA. Las CRO y los proveedores clínicos ofrecen soluciones web y en la nube para ensayos multicéntricos. El aumento de la investigación por contrato en oncología, neurología y enfermedades raras impulsa el crecimiento. La flexibilidad en la prestación de servicios atrae a clientes farmacéuticos de tamaño mediano y pequeño. Los informes y análisis avanzados mejoran la eficiencia de los ensayos. La expansión en los mercados emergentes impulsa la adopción. La formación y el soporte remoto reducen las barreras de implementación. La integración con los sistemas EDC y PRO garantiza un flujo de trabajo fluido. La seguridad de los datos y el cumplimiento normativo siguen siendo factores clave. La escalabilidad para ensayos globales acelera el uso. Las alianzas estratégicas con patrocinadores amplían el alcance del mercado. La innovación en la integración de dispositivos móviles y wearables impulsa una rápida adopción.
- Por plataforma
Sobre la base de la plataforma, el mercado está segmentado en Organizaciones de Investigación por Contrato, Compañías Farmacéuticas y Biofarmacéuticas, Fabricantes de Dispositivos Médicos, Hospitales y Laboratorios Clínicos, Compañías de Servicios de Consultoría, Investigación y Academia, y Otros. El segmento de Compañías Farmacéuticas y Biofarmacéuticas dominó con una participación en los ingresos del 44.7% en 2025, debido a los programas de desarrollo clínico a gran escala, la adopción de ensayos de medicina de precisión y la dependencia de datos electrónicos de resultados para presentaciones regulatorias. Las compañías farmacéuticas invierten en plataformas integradas de eCOA para estudios de oncología, cardiología y enfermedades crónicas. Los altos volúmenes de ensayos y las operaciones globales respaldan el dominio. La integración con EDC, PRO y dispositivos portátiles agiliza el flujo de trabajo. El cumplimiento normativo impulsa la adopción. La gestión centralizada de datos mejora la eficiencia de los ensayos. Las asociaciones a largo plazo con proveedores fortalecen el liderazgo. La implementación multidispositivo y multisitio respalda el uso. Las soluciones basadas en la nube mejoran la accesibilidad. La inversión en diagnósticos complementarios aumenta la utilización de la plataforma. Los informes en tiempo real mejoran la toma de decisiones. Los análisis avanzados aceleran los programas de desarrollo de fármacos.
Se espera que el segmento de Organizaciones de Investigación por Contrato (CRO) crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida del 15,8 % entre 2026 y 2033, impulsado por la externalización de ensayos clínicos, la mayor complejidad de los ensayos y la creciente demanda de una gestión integral de eCOA. Las CRO proporcionan plataformas eCOA escalables y basadas en la nube para estudios multinacionales. Su rápida adopción en los mercados emergentes acelera el crecimiento. La integración con los sistemas de los patrocinadores mejora la eficiencia operativa. Los análisis y paneles de control avanzados mejoran la información sobre los ensayos. Los modelos de implementación flexibles atraen a clientes farmacéuticos de tamaño mediano y pequeño. La formación y el soporte técnico facilitan la adopción. La alineación regulatoria global aumenta la confianza. La adopción de áreas multiterapéuticas impulsa la expansión. Los informes móviles y remotos aumentan el cumplimiento normativo. Las colaboraciones estratégicas con hospitales y el mundo académico fortalecen la presencia. En general, estos factores impulsan un sólido crecimiento.
Análisis regional del mercado de la evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia en Europa
- Se proyecta que el mercado europeo de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia se expandirá a una CAGR sustancial durante el período de pronóstico.
- Impulsado por la creciente adopción de soluciones de ensayos clínicos digitales, un estricto apoyo regulatorio y una creciente demanda de un seguimiento eficiente de los resultados de los pacientes.
- El crecimiento está impulsado por la integración de plataformas basadas en la nube, la recopilación de datos móviles y los resultados informados electrónicamente por los pacientes, junto con el enfoque de la región en mejorar la infraestructura de atención médica y la digitalización en la investigación clínica.
Evaluación electrónica de resultados clínicos (eCOA) del Reino Unido para el mercado de contenido con licencia
El mercado británico de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia dominó el mercado, con la mayor cuota de ingresos, de aproximadamente el 38,7 % en 2025. Este crecimiento se sustenta en una infraestructura sanitaria consolidada, una sólida adopción de tecnologías de ensayos clínicos digitales y un sólido respaldo regulatorio para el seguimiento electrónico de los resultados de los pacientes. Este crecimiento sustancial se debe a las innovaciones en plataformas en la nube, la recopilación de datos móviles y la integración fluida con sistemas centralizados de gestión de ensayos clínicos, lo que permite una medición más eficiente y precisa de los resultados de los pacientes.
Evaluación electrónica de resultados clínicos (eCOA) para el mercado de contenido con licencia en Alemania
Se prevé que el mercado alemán de evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia sea la región de mayor crecimiento durante el período de pronóstico, con una sólida tasa de crecimiento anual compuesta (TCAC) de dos dígitos. Este crecimiento se debe al aumento de la actividad de ensayos clínicos, el incremento del gasto sanitario, la ampliación del acceso a soluciones de salud digital y la creciente adopción de tecnologías de captura electrónica de datos y resultados informados por los pacientes en hospitales, centros de investigación y organizaciones farmacéuticas. Además, el énfasis de Alemania en la innovación tecnológica y la infraestructura sanitaria digital impulsa la rápida expansión del mercado.
Cuota de mercado de la evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia en Europa
La industria de la evaluación electrónica de resultados clínicos (eCOA) para contenido con licencia está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
• Medidata Solutions, Inc. (EE. UU.)
• CRF Health (Reino Unido)
• Signant Health (EE.
UU.) • Clinion ( EE. UU
.) • Celegence (EE. UU.
) • Icon plc (Irlanda ) •
PPD, Inc. (EE.
UU.) • Veeva Systems (EE. UU.)
• Oracle Health Sciences (EE. UU.)
• Synteract (EE. UU.)
• Bioclinica, Inc. (EE.
UU.) •
Complion (EE. UU.) • Curebase (EE. UU.)
• ClinOne
(EE. UU.) • CRF Bracket (EE. UU.)
• Datatrak International (EE. UU.)
• Electronic Data Capture, LLC (EE. UU.)
• PAREXEL International (EE. UU.)
• Health Solutions International (Reino Unido)
Últimos avances en la evaluación electrónica de resultados clínicos (eCOA) para el mercado de contenido con licencia en Europa
- En junio de 2023, ICON plc lanzó su Plataforma Digital ICON, una solución integrada que incluye aplicaciones móviles para pacientes, consentimiento electrónico, COA electrónico, captura directa de datos para servicios domiciliarios y gestión de tecnología sanitaria digital para optimizar la participación de los pacientes y la recopilación de datos en ensayos clínicos, lo que marca una importante expansión de las capacidades de COA en operaciones de ensayos clínicos digitales más amplias.
- En julio de 2023, Signant Health adquirió DSG, Inc., ampliando su cartera de tecnología de ensayos híbridos y eCOA para incluir capacidades EDC/DDC mejoradas y fortalecer su presencia en entornos de investigación clínica complejos, especialmente en Europa.
- En noviembre de 2023, Clinical Ink amplió sus soluciones de participación del paciente al integrar la herramienta de diagnóstico conductual SPUR con su conjunto de eCOA y biomarcadores digitales, ofreciendo conocimientos más profundos sobre el comportamiento del paciente y mejorando la evaluación holística de los resultados clínicos en todos los ensayos.
- En noviembre de 2024, uMotif completó la adquisición de ClinOne, combinando las capacidades de eCOA/ePRO con la productividad del sitio y las herramientas de gestión del consentimiento para crear una plataforma unificada que reduce los gastos administrativos y aumenta la eficiencia de la participación en los ensayos.
- En febrero de 2025, YPrime anunció el lanzamiento de su función de Formulario de cambio de datos automatizado (DCF) como parte de su plataforma eCOA 7.x, que ofrece una gestión de datos clínicos optimizada y eficiente y mejora la precisión en los flujos de trabajo de datos de ensayos.
- En 2025, Medable Inc. fue nombrada líder en la evaluación de la matriz PEAK de productos eCOA de ciencias biológicas del Everest Group, reconocida por la innovación en IA, el análisis en tiempo real y la captura de datos multimodales que agilizan las evaluaciones de resultados clínicos y mejoran la participación del paciente.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.