North America Exocrine Pancreatic Insufficiency Epi Therapeutics And Diagnostics Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
4.45 Billion
USD
7.82 Billion
2024
2032
USD
4.45 Billion
USD
7.82 Billion
2024
2032
| 2025 –2032 | |
| USD 4.45 Billion | |
| USD 7.82 Billion | |
|
|
|
|
تجزئة سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية، حسب التشخيص (فحوصات التصوير واختبارات وظائف البنكرياس)، والعلاج (إدارة التغذية، وعلاج تعويض إنزيم البنكرياس (PERT))، ونوع الدواء (جنيسة وعلامة تجارية)، والمستخدم النهائي (المستشفيات، والعيادات المتخصصة، والرعاية المنزلية، ومراكز التشخيص، والمعاهد البحثية والأكاديمية، وغيرها)، وقنوات التوزيع (العطاء المباشر، وصيدليات التجزئة، والموزعون الخارجيون، وغيرها) - اتجاهات الصناعة وتوقعاتها حتى عام 2032
حجم سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية
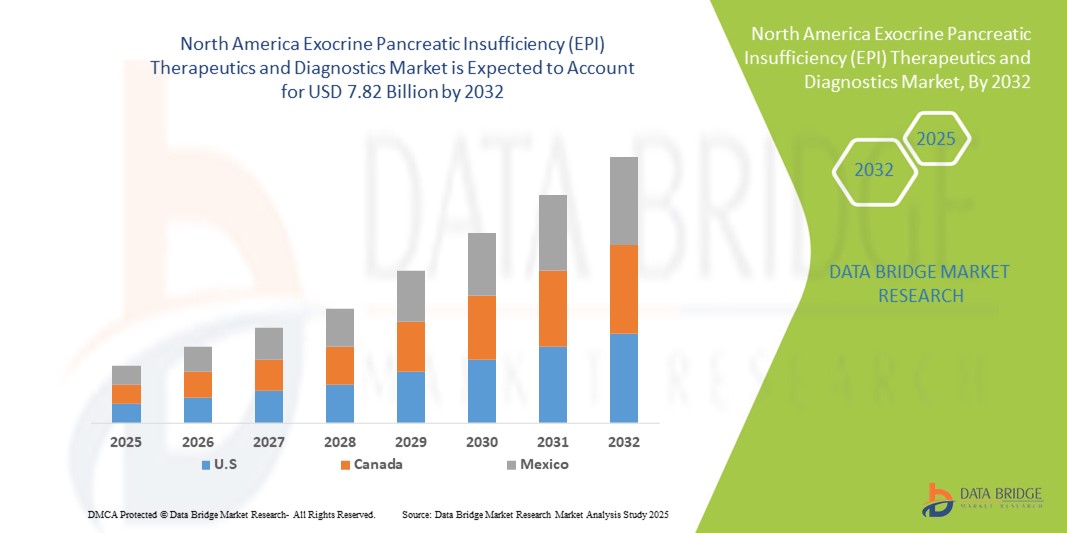
- تم تقييم حجم سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية بـ 4.45 مليار دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 7.82 مليار دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 7.3٪ خلال الفترة المتوقعة.
- إن نمو السوق مدفوع إلى حد كبير بالانتشار المتزايد لالتهاب البنكرياس المزمن والتليف الكيسي ، وهما السببان الرئيسيان لـ EPI، إلى جانب التقدم التكنولوجي في أدوات التشخيص والعلاجات الجديدة، مما يؤدي إلى تحسين إدارة المرض.
- علاوة على ذلك، فإن تزايد وعي المرضى والطلب على علاج استبدال إنزيم البنكرياس (PERT) الفعال وسهل الاستخدام والحلول التشخيصية ذات الصلة يُرسّخ هذه العلاجات كعلاج أساسي لـ EPI. تُسرّع هذه العوامل المتقاربة من اعتماد علاجات وتشخيصات EPI، مما يُعزز نمو هذا القطاع بشكل كبير.
تحليل سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
- تُعد العلاجات والتشخيصات لقصور البنكرياس الخارجي (EPI)، بما في ذلك العلاج باستبدال إنزيم البنكرياس (PERT) والاختبارات التشخيصية، مكونات حيوية بشكل متزايد للرعاية الصحية الحديثة لأمراض الجهاز الهضمي في كل من البيئات السريرية والمنزلية نظرًا لفعاليتها في إدارة سوء الامتصاص وتحسين نوعية حياة المريض والتكامل مع خطط العلاج الشخصية.
- الطلب المتزايد على علاجات وتشخيصات EPI مدفوع في المقام الأول بالانتشار المتزايد لالتهاب البنكرياس المزمن والتليف الكيسي واضطرابات البنكرياس الأخرى، والوعي المتزايد لدى المرضى، والتفضيل للعلاجات البديلة الفعالة سهلة الإدارة.
- سيطرت الولايات المتحدة على سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية بأكبر حصة إيرادات بلغت 79.2٪ في عام 2024، وتميزت بالتبني المبكر لأدوات التشخيص المتقدمة، والإنفاق المرتفع على الرعاية الصحية، والحضور القوي لشركات الأدوية والتكنولوجيا الحيوية الرئيسية، مع نمو كبير في العيادات والمستشفيات المتخصصة مدفوعًا بالابتكارات في تركيبات PERT والاختبارات التشخيصية.
- من المتوقع أن تكون كندا الدولة الأسرع نموًا في سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية خلال فترة التنبؤ بسبب زيادة الوعي باضطرابات البنكرياس وسياسات الرعاية الصحية المواتية والاستثمار المتزايد في البنية التحتية التشخيصية.
- هيمن قطاع العلاج باستبدال إنزيم البنكرياس (PERT) على سوق العلاجات والتشخيصات لقصور البنكرياس الخارجي (EPI) في أمريكا الشمالية بحصة سوقية بلغت 62.2% في عام 2024، مدفوعًا بفعاليته الراسخة في إدارة أعراض EPI والاعتماد السريري الواسع النطاق في المستشفيات ومرافق الرعاية المنزلية.
نطاق التقرير وتقسيم سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
|
صفات |
رؤى رئيسية حول سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
أمريكا الشمالية
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تشمل أيضًا تحليلًا متعمقًا من الخبراء وتحليل التسعير وتحليل حصة العلامة التجارية واستطلاع رأي المستهلكين وتحليل التركيبة السكانية وتحليل سلسلة التوريد وتحليل سلسلة القيمة ونظرة عامة على المواد الخام / المواد الاستهلاكية ومعايير اختيار البائعين وتحليل PESTLE وتحليل Porter والإطار التنظيمي. |
اتجاهات سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
رعاية المرضى المحسنة من خلال التشخيص المتقدم والعلاج الشخصي
- إن الاتجاه المهم والمتسارع في سوق العلاجات والتشخيصات EPI في أمريكا الشمالية هو التبني المتزايد لأدوات التشخيص المتقدمة وأنظمة العلاج باستبدال الإنزيم البنكرياسي (PERT) المخصصة، مما يؤدي إلى تحسين إدارة المرض ونتائج المرضى.
- على سبيل المثال، تسمح اختبارات الإيلاستاز البرازي والتنفس المتقدمة للأطباء بمراقبة تقدم EPI بدقة وتخصيص جرعات الإنزيم وفقًا لاحتياجات المريض الفردية
- يتيح دمج منصات الصحة الرقمية وأجهزة المراقبة القابلة للارتداء تتبع فعالية العلاج وتحسين الأعراض في الوقت الفعلي، مما يدعم خطط الرعاية الأكثر استباقية وشخصية
- تسمح هذه التقنيات بالتنسيق السلس بين أطباء الجهاز الهضمي ومقدمي الرعاية الأولية والمرضى، مما يسهل المراقبة عن بعد وتعديل العلاج الإنزيمي حسب الحاجة
- هذا التوجه نحو إدارة أكثر دقةً وقائمةً على البيانات ومُركزةً على المريض يُعيد صياغة توقعات رعاية EPI بشكل جذري. ونتيجةً لذلك، تُطوّر شركاتٌ مثل AbbVie وNestlé Health Science مجموعات تشخيصية وحلولًا علاجيةً مُحسّنةً بإمكانيات مراقبة مُحسّنة وجرعات مُخصصة لكل مريض.
- يتزايد الطلب على العلاجات والتشخيصات التي توفر المراقبة المتكاملة والجرعات الدقيقة وسهولة الاستخدام بسرعة في كل من البيئات السريرية والرعاية المنزلية، حيث يعطي المرضى والمقدمون الأولوية بشكل متزايد للفعالية وجودة الحياة
ديناميكيات سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
سائق
ارتفاع معدل انتشار اضطرابات البنكرياس وتزايد الوعي بها
- إن الانتشار المتزايد لالتهاب البنكرياس المزمن والتليف الكيسي واضطرابات البنكرياس ذات الصلة، إلى جانب الوعي المتزايد لدى المرضى والأطباء، هو محرك مهم لزيادة الطلب على علاجات وتشخيصات EPI
- على سبيل المثال، في مارس 2024، سلط تحديث سريري الضوء على النتائج المحسنة لدى المرضى الذين يتلقون جرعات PERT المُحسّنة، مما عزز أهمية التشخيص المبكر وتعديل العلاج
- مع إدراك المرضى ومقدمي الرعاية الصحية لفوائد تشخيص EPI في الوقت المناسب واستبدال الإنزيم الفعال، يزداد الطلب على التشخيص الدقيق والعلاج الفردي بشكل كبير
- علاوة على ذلك، فإن الشبكة المتوسعة من عيادات أمراض الجهاز الهضمي المتخصصة وبرامج دعم المرضى تعمل على دفع اعتماد PERT وأدوات التشخيص في كل من المستشفيات والمستشفيات الخارجية.
- إن سهولة إدارة PERT إلى جانب أدوات المراقبة، إلى جانب حملات التوعية المتزايدة وبرامج التعليم، تعمل على دفع المزيد من الإقبال على العلاجات والتشخيصات في جميع أنحاء أمريكا الشمالية
ضبط النفس/التحدي
تكاليف العلاج المرتفعة والتزام المرضى المحدود
- تشكل التكلفة المرتفعة نسبيًا لتركيبات الإنزيمات المتقدمة والاختبارات التشخيصية، وخاصة للعلاج طويل الأمد، تحديًا كبيرًا أمام تبني السوق الأوسع
- على سبيل المثال، يتوقف بعض المرضى عن استخدام علاج PERT أو يتناولون جرعة أقل من الجرعة المقررة بسبب النفقات المباشرة، مما يقلل من فعالية العلاج والالتزام به.
- إن معالجة القدرة على تحمل التكاليف من خلال التغطية التأمينية وبرامج مساعدة المرضى وخيارات الإنزيمات ذات التكلفة المنخفضة أمر بالغ الأهمية لتحسين الوصول والامتثال
- بالإضافة إلى ذلك، فإن عدم التزام المريض والإدارة غير السليمة للإنزيمات يمكن أن يحد من فعالية العلاج، مما يؤكد الحاجة إلى الدعم التعليمي وأدوات المراقبة لضمان الاستخدام الصحيح
- في حين تتزايد الوعي وخيارات العلاج، فإن التعقيد المتصور وتكلفة العلاج لا يزالان يعيقان تبني بعض المرضى لهذا العلاج، وخاصة في المناطق ذات الموارد الصحية المحدودة.
- إن التغلب على هذه التحديات من خلال استراتيجيات خفض التكاليف وتثقيف المرضى وحلول المراقبة الرقمية سيكون أمرًا حيويًا لتحقيق نمو مستدام للسوق وتحسين نتائج المرضى
نطاق سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية
يتم تقسيم السوق على أساس التشخيص والعلاج ونوع الدواء والمستخدم النهائي وقناة التوزيع.
- حسب التشخيص
بناءً على التشخيص، يُقسّم سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية إلى اختبارات تصويرية واختبارات وظائف البنكرياس. وقد هيمن قطاع اختبارات وظائف البنكرياس على السوق محققًا أكبر حصة من الإيرادات في عام 2024، مدفوعًا بدوره الأساسي في التشخيص الدقيق لقصور الإنزيم وتوجيه العلاج ببدائل إنزيم البنكرياس (PERT). غالبًا ما يُفضّل الأطباء اختبارات تحفيز الإيلاستاز والسيكريتين في البراز لحساسيتها العالية وخصوصيتها. ويستفيد هذا القطاع من تنامي الوعي بـ EPI بين المرضى ومقدمي الرعاية الصحية. كما تدعم الاختبارات غير الجراحية والاختبارات التي تُجرى في نقطة الرعاية التبني القوي لها في المستشفيات والعيادات التخصصية ومراكز التشخيص. ويُعزز ارتفاع معدل انتشار التهاب البنكرياس المزمن والتليف الكيسي استخدام اختبارات وظائف البنكرياس. كما تُسهم التحسينات التكنولوجية في دقة الاختبارات وسرعة إنجازها في تعزيز هيمنتها.
من المتوقع أن يشهد قطاع اختبارات التصوير أسرع نمو خلال فترة التوقعات، مدفوعًا بالتطورات في التصوير بالرنين المغناطيسي، والتصوير المقطعي المحوسب، والتصوير بالموجات فوق الصوتية بالمنظار. تساعد اختبارات التصوير على الكشف عن التشوهات الهيكلية في البنكرياس وأسبابها الكامنة، مثل التهاب البنكرياس المزمن. يتيح التكامل المتزايد مع منصات الطب عن بُعد استخدامًا أوسع في العيادات الخارجية والرعاية المنزلية. كما أن تزايد توفر معدات التصوير وتحسين دقتها يُسرّع من اعتمادها. ويعتمد الأطباء بشكل متزايد على اختبارات التصوير لمراقبة تطور المرض وفعالية العلاج. ويدعم هذا النمو أيضًا قيام المستشفيات والعيادات المتخصصة بتحديث بنيتها التحتية التشخيصية.
- حسب العلاج
بناءً على العلاج، يُقسّم سوق علاجات وتشخيصات قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية إلى إدارة التغذية وعلاج تعويض إنزيم البنكرياس (PERT). هيمن قطاع PERT على السوق في عام 2024 بحصة سوقية بلغت 62.2% بفضل فعاليته المُثبتة في إدارة أعراض قصور البنكرياس الخارجي، مثل سوء الامتصاص ونقص العناصر الغذائية. يُفضّل PERT على نطاق واسع لجرعاته المُوحدة، وسهولة إعطائه، ونتائجه السريرية الإيجابية. تُعدّ المستشفيات والعيادات التخصصية ومؤسسات الرعاية المنزلية من أبرز المستخدمين النهائيين. تُعزز الابتكارات، مثل تركيبات الإطلاق المُؤجل، وتقليل حجم الكبسولات، والعلاجات المُركّبة، التزام المرضى بالعلاج. كما تُعزز برامج التسويق القوية ودعم المرضى التي تُقدمها شركات الأدوية هذا القطاع. كما يُسهم تزايد وعي المرضى والأطباء بأهمية تعويض الإنزيم المُناسب في تعزيز هيمنته.
من المتوقع أن يُسجل قطاع إدارة التغذية أسرع نمو خلال فترة التوقعات، مدفوعًا بالدور المتزايد للتدخلات الغذائية في دعم علاج EPI. ويتزايد استخدام المكملات الغذائية، والأنظمة الغذائية عالية السعرات الحرارية، والفيتامينات التي تذوب في الدهون، إلى جانب برنامج PERT. ويعزز تثقيف المرضى والبرامج التي يُشرف عليها أخصائيو التغذية في المستشفيات ومرافق الرعاية المنزلية من تبني هذا القطاع. كما يُعزز دمج التغذية مع خطط العلاج الشخصية فعالية العلاج. ويشجع الوعي المتزايد بتأثير النظام الغذائي على إدارة الأعراض على النمو. ويتسارع تبني حلول إدارة التغذية في الرعاية المنزلية والعيادات الخارجية.
- حسب نوع الدواء
بناءً على نوع الدواء، يُقسّم سوق علاجات وتشخيصات قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية إلى أدوية عامة وأدوية ذات علامات تجارية. هيمنت الأدوية ذات العلامات التجارية على السوق في عام 2024 بفضل قوة التحقق السريري، والموافقات التنظيمية، وثقة المرضى. توفر منتجات PERT ذات العلامات التجارية نشاطًا إنزيميًا موحدًا وتغليفًا متناسقًا. تعزز برامج دعم المرضى وحملات التوعية الالتزام بالعلاج. تُستخدم الأدوية ذات العلامات التجارية على نطاق واسع في المستشفيات والعيادات التخصصية وخدمات الرعاية المنزلية. تروج شركات الأدوية بنشاط لهذه المنتجات، مما يعزز هيمنتها على السوق. كما أن الأدلة السريرية وتفضيلات الأطباء تدعم هذا القطاع بشكل أكبر.
من المتوقع أن يشهد قطاع الأدوية الجنيسة أسرع نمو خلال فترة التوقعات، مدفوعًا بحساسية التكلفة وزيادة التغطية التأمينية. تتميز منتجات PERT الجنيسة بفعالية مماثلة للأدوية ذات العلامات التجارية وبأسعار أقل. ويتزايد الإقبال على استخدامها في المستشفيات العامة، وخدمات الرعاية المنزلية، والعيادات الخارجية. كما تدعم المبادرات الحكومية التي تشجع على توفير العلاج بأسعار معقولة هذا النمو. كما تعمل الأدوية الجنيسة على توسيع نطاق الوصول إلى الفئات المحرومة من الخدمات. ويساهم تزايد الوعي بالخيارات الفعالة من حيث التكلفة في تسريع اعتمادها في أمريكا الشمالية.
- حسب المستخدم النهائي
بناءً على المستخدم النهائي، يُقسّم سوق علاجات وتشخيصات قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية إلى مستشفيات، وعيادات متخصصة، ومراكز رعاية منزلية، ومراكز تشخيص، ومعاهد بحثية وأكاديمية، وغيرها. هيمن قطاع المستشفيات على السوق في عام 2024 بفضل تركيزه على مرافق التشخيص المتقدمة وخدمات إدارة PERT. تقدم المستشفيات رعاية للمرضى الداخليين والخارجيين المصابين بقصور البنكرياس الإفرازي. كما يُعزز التعاون الوثيق مع شركات الأدوية من تبني هذه الخدمات. كما تُعدّ المستشفيات مراكز رئيسية لتثقيف المرضى ومتابعة العلاج. يستفيد هذا القطاع من انتشار التهاب البنكرياس المزمن والتليف الكيسي. ويُسهم توسع أقسام أمراض الجهاز الهضمي والعيادات المتخصصة في تعزيز هيمنته.
من المتوقع أن يشهد قطاع الرعاية المنزلية أسرع نمو خلال فترة التوقعات، مدفوعًا بالرعاية التي تركز على المريض وإدارة العلاج عن بُعد. ويتم تسهيل تبني الرعاية المنزلية من خلال منصات الطب عن بُعد وأدوات المراقبة الرقمية. ويمكن للمرضى تلقي برنامج PERT والإرشادات الغذائية من منازلهم براحة تامة. كما تدعم الرعاية المنزلية الالتزام بالرعاية من خلال الإشراف السريري عن بُعد. ويشجع زيادة الوعي بإدارة برنامج EPI خارج المستشفيات على النمو. وتُعد الراحة وتقليل زيارات المستشفيات من العوامل الرئيسية التي تدفع تبني هذا القطاع.
- حسب قناة التوزيع
بناءً على قنوات التوزيع، يُقسّم سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في أمريكا الشمالية إلى مناقصة مباشرة، وصيدليات تجزئة، وموزعين تابعين لجهات خارجية، وغيرها. هيمن قطاع صيدليات التجزئة على السوق في عام 2024 بفضل سهولة الوصول إليه، وسهولة الوصول إليه، والتواجد القوي لمنتجات PERT ذات العلامات التجارية. تقدم الصيدليات خدمات استشارية ودعم لضمان الالتزام. تُحسّن الشراكات بين شركات الأدوية وسلاسل البيع بالتجزئة من توافر الأدوية. يستفيد المرضى من سهولة صرف الوصفات الطبية والوصول إليها في الوقت المناسب. تُستخدم صيدليات التجزئة على نطاق واسع في جميع أنحاء الولايات المتحدة وكندا. يُعزز التوسع المستمر لشبكات الصيدليات من هيمنتها.
من المتوقع أن يشهد قطاع المناقصات المباشرة أسرع نمو خلال فترة التوقعات، مدفوعًا بالمشتريات الجماعية من المستشفيات والعيادات التخصصية والبرامج الحكومية. توفر المناقصات المباشرة حلولاً فعّالة من حيث التكلفة لأنظمة PERT والتشخيص. ويمكن للمشترين المؤسسيين إدارة أعداد كبيرة من المرضى بكفاءة. تدعم سلاسل التوريد المُبسّطة اعتماد هذه الأنظمة في المستشفيات والعيادات. كما يُسرّع الطلب المتزايد من مبادرات الرعاية الصحية العامة النمو. ويدعم هذا النمو أيضًا العقود طويلة الأجل التي تضمن توافرًا ثابتًا للمنتجات.
تحليل إقليمي لسوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
- سيطرت الولايات المتحدة على سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية بأكبر حصة إيرادات بلغت 79.2٪ في عام 2024، والتي تميزت بالتبني المبكر لأدوات التشخيص المتقدمة، والإنفاق المرتفع على الرعاية الصحية، والحضور القوي لشركات الأدوية والتكنولوجيا الحيوية الرئيسية، مع نمو كبير في العيادات والمستشفيات المتخصصة مدفوعة بالابتكارات في تركيبات PERT والاختبارات التشخيصية.
- يقدر المرضى ومقدمو الرعاية الصحية في المنطقة بشكل كبير إمكانية الوصول إلى علاج استبدال إنزيم البنكرياس (PERT)، والاختبارات التشخيصية المتقدمة مثل الإيلاستاز البرازي واختبارات التصوير، وخطط العلاج المتكاملة التي تعمل على تحسين نتائج المرضى ونوعية الحياة.
- يتم دعم هذا التبني الواسع النطاق من خلال البنية التحتية الراسخة للرعاية الصحية، والإنفاق المرتفع على الرعاية الصحية، والسكان المتقدمين من الناحية التكنولوجية، والتفضيل المتزايد لنماذج الرعاية التي تركز على المريض، مما يجعل العلاجات والتشخيصات EPI حلولاً أساسية في كل من المستشفيات ومرافق الرعاية المنزلية.
نظرة عامة على سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI )
استحوذ سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في الولايات المتحدة على أكبر حصة من الإيرادات في أمريكا الشمالية عام 2024، مدفوعًا بالانتشار الواسع لالتهاب البنكرياس المزمن، والتليف الكيسي، واضطرابات البنكرياس الأخرى. ويولي المرضى ومقدمو الرعاية الصحية أولوية متزايدة للتشخيص المبكر والعلاج الفعال ببدائل إنزيم البنكرياس (PERT) لتحسين النتائج وجودة الحياة. كما أن الإقبال المتزايد على أدوات التشخيص المتكاملة، وأنظمة مراقبة المرضى، وعلاجات الرعاية المنزلية يُعزز نمو السوق. علاوة على ذلك، تُسهم البنية التحتية السريرية المتطورة، والتغطية التأمينية الواسعة، ووجود شركات الأدوية والتكنولوجيا الحيوية الرائدة بشكل كبير في توسع السوق. كما يُعزز اعتماد التطبيب عن بُعد ومنصات الصحة الرقمية لإدارة العلاج عن بُعد من إمكانية الوصول والالتزام.
نظرة عامة على سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في كندا
من المتوقع أن يشهد سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في كندا نموًا بمعدل نمو سنوي مركب كبير خلال فترة التوقعات، مدفوعًا بشكل رئيسي بتزايد الوعي باضطرابات البنكرياس وزيادة الاستثمار في البنية التحتية للرعاية الصحية. تُعزز برامج الرعاية الصحية الحكومية وسياسات السداد المواتية اعتماد كل من التشخيص والعلاج. ينجذب المرضى والأطباء الكنديون إلى فوائد اختبار PERT، واختبارات وظائف البنكرياس المتقدمة، وحلول إدارة التغذية. يشهد السوق نموًا في المستشفيات والعيادات التخصصية ومؤسسات الرعاية المنزلية، مع التركيز بشكل رئيسي على التشخيص المبكر والعلاج الشخصي. كما تُشجع المبادرات التعليمية وبرامج دعم المرضى المتزايدة على زيادة الإقبال على السوق.
نظرة عامة على سوق علاجات وتشخيص قصور البنكرياس الإفرازي (EPI) في المكسيك
من المتوقع أن يشهد سوق علاجات وتشخيصات قصور البنكرياس الإفرازي (EPI) في المكسيك نموًا ملحوظًا بمعدل نمو سنوي مركب خلال الفترة المتوقعة، مدفوعًا بزيادة الإنفاق على الرعاية الصحية وتزايد وعي المرضى بـ EPI. وتدعم الجهود المبذولة لتوسيع نطاق الوصول إلى الاختبارات التشخيصية وتركيبات PERT بأسعار معقولة نمو السوق. وتعتمد المستشفيات والعيادات التخصصية بشكل متزايد خيارات تشخيصية وعلاجية متقدمة، بينما يبرز العلاج المنزلي كحل مناسب للمرضى في المناطق النائية. ويعزز التعاون مع شركات الأدوية والحملات التثقيفية التشخيص المبكر والالتزام بالعلاج. كما يُتوقع أن تواصل المبادرات الحكومية لتعزيز البنية التحتية للرعاية الصحية تحفيز نمو السوق.
حصة سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية
إن صناعة علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية يقودها في المقام الأول شركات راسخة، بما في ذلك:
- شركة AbbVie Inc. (الولايات المتحدة)
- شركة إنترو ثيرابيوتكس (الولايات المتحدة)
- شركة أكسوفانت للعلاجات الجينية المحدودة (الولايات المتحدة)
- شركة كمبرلاند للأدوية (الولايات المتحدة)
- بروتاليكس بيوثيرابيوتكس، المحدودة (الولايات المتحدة)
- شركة بروميتيك لعلوم الحياة (كندا)
- شركة أبتاليس فارما (كندا)
- شركة إنزو بيوكيم (الولايات المتحدة)
- شركة ثيرمو فيشر العلمية (الولايات المتحدة)
- مختبرات بيو-راد، المحدودة (الولايات المتحدة)
- أبوت (الولايات المتحدة)
- شركة إف. هوفمان-لا روش المحدودة (الولايات المتحدة)
- شركة سيمنز هيلثينيرز إيه جي (ألمانيا)
- شركة بايو-تكني (الولايات المتحدة)
- بيركين إلمر (الولايات المتحدة)
- شركة لومينا (الولايات المتحدة)
- شركة أجيلنت تكنولوجيز (الولايات المتحدة)
- شركة ميرك وشركاه المحدودة (الولايات المتحدة)
ما هي التطورات الأخيرة في سوق علاجات وتشخيص قصور البنكرياس الخارجي (EPI) في أمريكا الشمالية؟
- في مايو 2024، طُوّرت أداة جديدة لتقييم الأعراض، وهي EPI/PEI-SS، من خلال مبادرة يقودها المرضى للمساعدة في فحص قصور البنكرياس الخارجي. تهدف هذه الأداة، التي وُضعت من خلال مراجعة الأدبيات المتوفرة وقوائم الأعراض التي أعدها المرضى، إلى توفير طريقة أكثر شمولاً لتقييم تواتر وشدة أعراض قصور البنكرياس الخارجي الشائعة.
- في ديسمبر 2023، أبرمت كودكسيس ونستله هيلث ساينس اتفاقية شراء لـ CDX-7108، وهو علاج إنزيمي فموي جديد لـ EPI. بموجب شروط الاتفاقية، ستكون نستله هيلث ساينس مسؤولة عن التطوير المستمر لـ CDX-7108 وتسويقه تجاريًا، بما في ذلك جميع التكاليف المرتبطة به.
- في مايو 2023، أكملت شركة فيرست ويف بيوفارما فحص المرضى في المرحلة الثانية من تجربتها السريرية SPAN لدواء أدروليباز لعلاج EPI لدى مرضى التليف الكيسي. يمثل هذا الإنجاز خطوة مهمة في تقييم إمكانات أدروليباز كخيار علاجي لـ EPI، ومن المتوقع أن تُسهم نتائجه في توجيه استراتيجيات العلاج المستقبلية.
- في فبراير 2023، أعلنت كودكسيس ونستله هيلث ساينس عن نتائج أولية من المرحلة الأولى من تجربة سريرية لـ CDX-7108، وهو علاج إنزيمي فموي جديد لعلاج التهاب السحايا البكتيري. هدفت التجربة إلى تقييم سلامة CDX-7108 وتحمله وحركيته الدوائية لدى الأشخاص الأصحاء. يمثل هذا التعاون خطوة مهمة في تطوير علاجات إنزيمية غير مشتقة من الخنازير لمرضى التهاب السحايا البكتيري.
- في أغسطس 2021، أعلنت شركة AzurRx Biopharma، Inc. (المعروفة الآن باسم First Wave BioPharma، Inc.) عن اكتمال التسجيل في تجربتها السريرية للمرحلة الثانية من MS1819 بالاشتراك مع العلاج باستبدال إنزيم البنكرياس (PERT) لعلاج قصور البنكرياس الخارجي الشديد (EPI) لدى مرضى التليف الكيسي.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.