سوق الكشف عن جينات CRISPR وتشخيصها في أمريكا الشمالية، حسب الفئة (الفئة 1 - بروتينات متعددة التأثير والفئة 2 - بروتين رابط CrRNA واحد)، المنتجات والخدمات (المنتجات والخدمات)، التطبيق (التشخيصات الطبية الحيوية، هندسة الجينوم، اكتشاف الأدوية، التطبيقات الزراعية وغيرها)، سير العمل (إعداد العينة، التضخيم المسبق، CrRNA، إنزيمات Cas والاستشعار)، المستخدم النهائي (المستشفيات ومراكز التشخيص وشركات التكنولوجيا الحيوية والمعاهد الأكاديمية والبحثية وغيرها)، قناة التوزيع (العطاء المباشر، مبيعات التجزئة) اتجاهات الصناعة والتوقعات حتى عام 2029
تعريف السوق ورؤيته
إن تقنية كريسبر عبارة عن تكرارات قصيرة متناوبة منتظمة التباعد وهي أداة لتحرير الجينوم، فهي تسمح للباحثين بتغيير تسلسلات الحمض النووي وتعديل وظيفة الجينات بسهولة. ولديها العديد من التطبيقات المحتملة، بما في ذلك تصحيح العيوب الجينية وعلاج الأمراض ومنع انتشارها. وقد تم استخدام التشخيصات القائمة على كريسبر في العديد من التطبيقات الطبية الحيوية، مثل استشعار المؤشرات الحيوية القائمة على الأحماض النووية للأمراض المعدية وغير المعدية والكشف عن الأمراض الوراثية. تتكون مجموعات التحليل في كريسبر من مكونين: بروتين يسمى Cas9 وRNA دليل، وهو سلسلة من جزيئات الأحماض النووية ذات شفرة وراثية معينة.
تم تعديل نظام CRISPR-Cas9 هذا لاستخدامه في الخلايا الثديية. يمكننا إما تعطيل جينات معينة عن طريق إدخال تسلسل دليل (sgRNA) خاص بالجين الذي يهمنا عن طريق إدخال طفرات إطارية عبر الانضمام النهائي غير المتجانس (NHEJ) أو توليد طفرات إدخال.
لقد وسعت أنظمة CRISPR-Cas 9 نطاق التشخيص والخدمات في علاجات الجينات والخلايا. وتستثمر شركات الأدوية بكثافة في البحث والتطوير لتطوير منتجات جديدة، مع زيادة كبيرة في عوامل علاج الجينات والخلايا التي تدخل مرحلة التطوير المبكر. ومن شأن استثمار اللاعبين في السوق أن يسمح بإنتاج علاجات آمنة وفعالة للمرضى الذين هم في حاجة ماسة إليها.
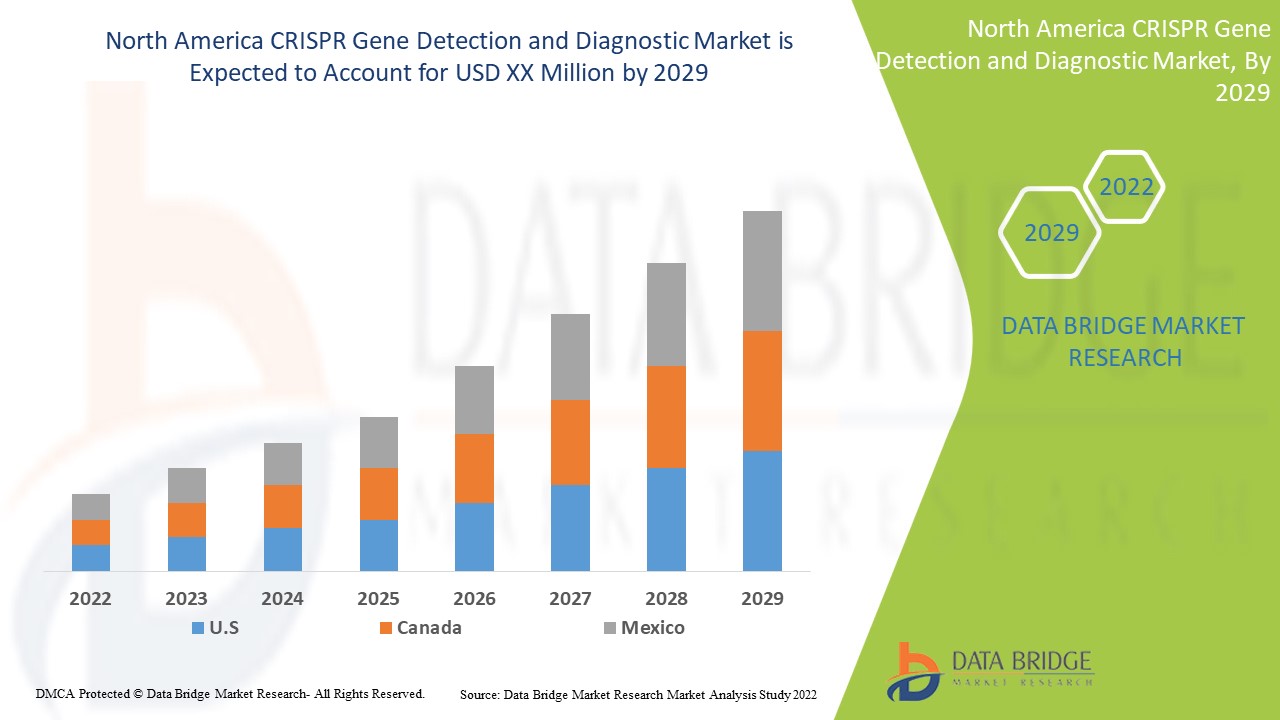
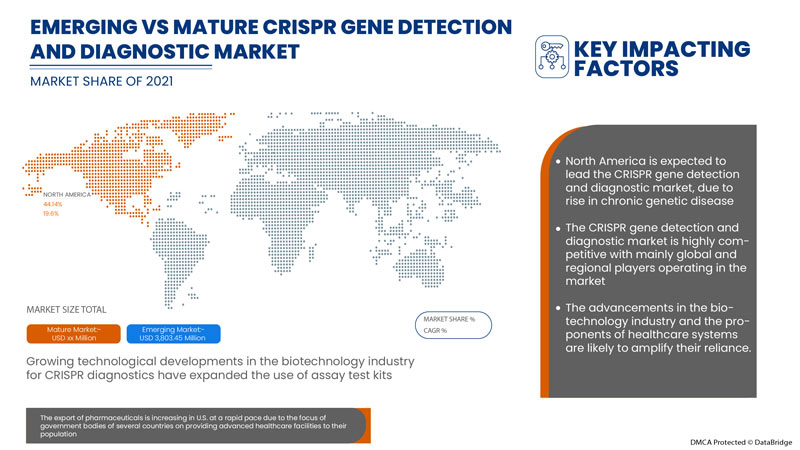
إن الكشف عن جين CRISPR وتشخيصه في أمريكا الشمالية داعم ويهدف إلى تقليل شدة الأعراض. تحلل شركة Data Bridge Market Research أن سوق الكشف عن جين CRISPR وتشخيصه سينمو بمعدل نمو سنوي مركب يبلغ 19.6٪ خلال الفترة المتوقعة من 2022 إلى 2029.
|
تقرير القياس |
تفاصيل |
|
فترة التنبؤ |
2022 إلى 2029 |
|
سنة الأساس |
2021 |
|
سنوات تاريخية |
2020 (قابلة للتخصيص حتى 2019 - 2014) |
|
وحدات كمية |
الإيرادات بالملايين من الدولارات الأمريكية، التسعير بالدولار الأمريكي |
|
القطاعات المغطاة |
حسب الفئة (الفئة 1 - بروتينات متعددة الفعالية والفئة 2 - بروتين رابط CrRNA واحد)، المنتجات والخدمات (المنتجات والخدمات)، التطبيق (التشخيصات الطبية الحيوية، هندسة الجينوم، اكتشاف الأدوية، التطبيقات الزراعية وغيرها)، سير العمل (إعداد العينة، التضخيم المسبق، CrRNA، إنزيمات Cas والاستشعار)، المستخدم النهائي (المستشفيات ومراكز التشخيص وشركات التكنولوجيا الحيوية والمعاهد الأكاديمية والبحثية وغيرها) قناة التوزيع (العطاء المباشر، مبيعات التجزئة) |
|
الدول المغطاة |
الولايات المتحدة وكندا والمكسيك |
|
الجهات الفاعلة في السوق المشمولة |
GenScript، OriGene Technologies, Inc.، Applied StemCell، GeneCopoeia, Inc.، Agilent Technologies, Inc.، Synthego، BioVision Inc.، Hera Biolabs، Cellecta, Inc.، New England Biolabs، 10x Genomics، addgene CasTag Biosciences، Merck KGaA، Integrated DNA Technologies, Inc. (شركة تابعة لشركة Danaher)، Thermo Fisher Scientific Inc. وغيرها |
ديناميكيات سوق الكشف عن الجينات وتشخيصها باستخدام تقنية CRISPR في أمريكا الشمالية
السائقين
- ارتفاع معدل انتشار الأمراض المزمنة
الأمراض المزمنة هي حالات صحية شائعة، حيث يعاني واحد من كل ثلاثة بالغين من حالات مزمنة. وقد أثرت الأمراض المزمنة على صحة ونوعية حياة العديد من المواطنين.
يُختصر مصطلح CRISPR على أنه تكرارات قصيرة منتظمة متباعدة. وفي السنوات الأخيرة، أصبح CRISPR أداة قيادية لتحرير الجينات، والتي تُستخدم لتغيير تسلسلات معينة من الحمض النووي في الخلية. ولـ CRISPR استخدام مهم في البحث عن وعلاج مرض هنتنغتون، وضمور العضلات، والسرطان، وارتفاع نسبة الكوليسترول.
على سبيل المثال،
- في عام 2021، ذكرت بيانات منظمة NORD - المنظمة الوطنية للاضطرابات النادرة، أن معدل الإصابة بضمور العضلات دوشين (DMD) هو حالة وراثية شائعة، تؤثر على 1 من كل 3500 مولود ذكر في جميع أنحاء العالم.
- ارتفاع الاستثمار في البحث والتطوير
لقد أدت تقنيات تحرير الجينات، مثل نظام CRISPR-Cas 9، إلى توسيع نطاق التشخيص والخدمات في مجال علاجات الجينات والخلايا. وتستثمر شركات الأدوية بكثافة في البحث والتطوير لتطوير منتجات جديدة، مع زيادة كبيرة في عوامل علاج الجينات والخلايا التي تدخل مرحلة التطوير المبكر. ومن شأن استثمار اللاعبين في السوق أن يسمح بتحقيق هدف إنتاج علاجات آمنة وفعالة للمرضى المحتاجين بشدة.
على سبيل المثال،
- في فبراير 2022، جمعت شركة سينثيغو 200 مليون دولار أمريكي كاستثمار للبحث والتطوير لتعزيز تطوير الأدوية القائمة على تقنية كريسبر من مرحلة البحث المبكرة إلى العيادة. ستستخدم سينثيغو مبلغ الاستثمار من تمويل السلسلة E لتسريع إنشاء تشخيصات وخدمات كريسبر
توفر التمويل لتشخيص جين CRISPR
يتم تمويل تشخيص وأبحاث جينات CRISPR من ميزانية المعهد الوطني للصحة (NIH). يمول القطاع الخاص أيضًا اكتشاف جينات CRISPR والبحث عنها، ولكن مثل هذا الاستثمار يحدث عمومًا في وقت لاحق، أثناء مرحلة الاختبار والتطوير، ثم أثناء البحث الأساسي الأولي. نظرًا لأن تحرير الجينوم هو مجال جديد، فيجب أن تشرف عليه هيئة حكومية غير متحيزة؛ إدارة الغذاء والدواء حذرة ودقيقة، لكنها تكافح بلا نهاية للحصول على التمويل، مما يجعل الاستثمار طويل الأجل الذي يتماشى مع الدفع للمستفيدين المحتملين في المستقبل، سيعزز بشكل أكبر نمو سوق اكتشاف وتشخيص جينات CRISPR.
علاوة على ذلك، فإن التقدم في تشخيص جينات CRISPR، والمبادرات المتزايدة من قبل المنظمات العامة والخاصة لنشر الوعي والتمويل الحكومي المتزايد هي العوامل التي ستوسع سوق اكتشاف جينات CRISPR. عوامل أخرى مثل زيادة الطلب على العلاجات الفعالة وزيادة الوعي بالتشخيص في الوقت المناسب ستؤثر بشكل إيجابي على معدل نمو سوق اكتشاف جينات CRISPR والتشخيص. بالإضافة إلى ذلك، فإن الدخل المرتفع المتاح، والعدد المتزايد من الأمراض المزمنة، وتغيير نمط الحياة سيؤدي إلى توسع سوق اكتشاف جينات CRISPR والتشخيص.
فرص
- ارتفاع الإنفاق على الرعاية الصحية
علاوة على ذلك، فإن زيادة أنشطة البحث والتطوير وزيادة الاستثمارات من قبل الحكومة والمنظمات الخاصة من شأنها أن تعزز فرصًا جديدة لمعدل نمو السوق.
- المبادرة الاستراتيجية من قبل اللاعبين في السوق
لقد زاد الطلب على اكتشاف جين CRISPR وتشخيصه في الولايات المتحدة الأمريكية، وذلك بفضل العلاج في الوقت المناسب للحالات المزمنة. تعمل هذه العوامل المواتية على تعزيز الحاجة إلى الأدوية، ولتحقيق الطلب في السوق، يستخدم اللاعبون الصغار والكبار في السوق استراتيجيات مختلفة.
ويحاول اللاعبون الرئيسيون أيضًا ابتكار استراتيجيات محددة، مثل إطلاق المنتجات، والاستحواذات، والموافقات، والتوسعات، والشراكات، لضمان حسن سير الأعمال، وتجنب المخاطر، وزيادة النمو طويل الأجل في مبيعات السوق.
على سبيل المثال،
- في مايو 2021، قامت شركة Horizon Discovery Ltd. بتوسيع محفظة تعديل الجينات من خلال أول دليل RNA اصطناعي أحادي ومثبط dcas9 قيد براءة اختراع للتدخل في CRISPR في Waltham. أدى توسيع المحفظة إلى زيادة مبيعات وإيرادات محفظة الدليل RNA الاصطناعي في جميع أنحاء الولايات المتحدة ومنطقة المملكة المتحدة وزاد من التعاون مع اللاعبين في السوق
كما أن إطلاق العلاجات الفعالة والتجارب السريرية المستمرة من شأنه أن يوفر فرصًا مفيدة لسوق الكشف عن جينات CRISPR وتشخيصها في الفترة المتوقعة 2022-2029. كما أن الحاجة العالية غير الملباة للتطورات الحالية في تكنولوجيا الرعاية الصحية من شأنها أن تزيد من معدل نمو سوق الكشف عن جينات CRISPR وتشخيصها في المستقبل.
القيود/التحديات
ومع ذلك، فإن التكلفة العالية لتشخيصات CRISPR والمخاطر التي تواجهها أثناء استخدام تشخيصات CRISPR ستعيق معدل نمو سوق الكشف عن جينات CRISPR والتشخيص. بالإضافة إلى ذلك، فإن المخاطر التي تحدث أثناء استخدام أجهزة التصوير بالرنين المغناطيسي ستعيق نمو سوق الكشف عن جينات CRISPR والتشخيص. إن الافتقار إلى الخبرة الماهرة واللوائح من شأنه أن يشكل تحديًا أكبر للسوق في فترة التنبؤ المذكورة أعلاه.
- ارتفاع تكلفة التشخيصات القائمة على تقنية كريسبر
إن الإمكانات الهائلة للعلاجات القائمة على تقنية كريسبر تأتي مصحوبة بتكلفة باهظة. حيث تتطلب علاجات تحرير الجينوم القصوى قدرًا متزايدًا من الوقت للتطوير والإنتاج، ومن ثم تحدث زيادة في التكلفة. علاوة على ذلك، فإن مجموعات التحليل والأدوية المتعلقة باكتشاف جينات كريسبر وتشخيصها قابلة للتطبيق على شريحة كبيرة من السكان. ويتم فرض هذه التكاليف على المرضى. لذلك، من المتوقع أن تظهر التكلفة المرتفعة الحالية اتجاهًا تنازليًا في المستقبل.
على سبيل المثال،
- في يوليو 2021، وفقًا لشركة Integrated DNA Technologies, Inc.، فإن أول اختبار تشخيصي قائم على CRISPR متاح تجاريًا لفيروس SARS-CoV-2 بما في ذلك LAMP النسخ العكسي (RT-LAMP) كتضخيم مسبق متاح حاليًا بسعر 30.15 دولارًا أمريكيًا لكل تفاعل
يقدم تقرير سوق الكشف عن جينات CRISPR والتشخيص تفاصيل عن التطورات الحديثة الجديدة واللوائح التجارية وتحليل الاستيراد والتصدير وتحليل الإنتاج وتحسين سلسلة القيمة وحصة السوق وتأثير اللاعبين المحليين والمحليين في السوق وتحليل الفرص من حيث جيوب الإيرادات الناشئة والتغيرات في لوائح السوق وتحليل نمو السوق الاستراتيجي وحجم السوق ونمو سوق الفئات ومنافذ التطبيق والهيمنة وموافقات المنتجات وإطلاق المنتجات والتوسعات الجغرافية والابتكارات التكنولوجية في السوق. للحصول على مزيد من المعلومات حول سوق الكشف عن جينات CRISPR والتشخيص، اتصل بـ Data Bridge Market Research للحصول على موجز محلل، وسيساعدك فريقنا في اتخاذ قرار سوقي مستنير لتحقيق نمو السوق.
تحليل وبائيات المرضى
وفقًا لدراسة أجرتها شركة غلوبوكان، في عام 2020، كان سرطان الثدي هو الأكثر انتشارًا بين المصابين، بنحو 11.7%، يليه سرطان الرئة بنسبة 11.40%، وسرطان القولون والمستقيم بنسبة 10.00%، وسرطان عنق الرحم والمريء بنسبة أقل من حالات الإصابة.
كما يوفر لك سوق الكشف عن جينات CRISPR والتشخيص تحليلًا تفصيليًا للسوق لتحليل المرضى والتشخيص والعلاج. الانتشار، والوقوع، والوفيات، ومعدلات الالتزام هي بعض متغيرات البيانات المتوفرة في التقرير. يتم تحليل تحليلات التأثير المباشر أو غير المباشر لعلم الأوبئة على نمو السوق لإنشاء نموذج إحصائي متعدد المتغيرات أكثر قوة وشمولاً للتنبؤ بالسوق في فترة النمو.
تأثير COVID-19 على سوق الكشف عن الجينات وتشخيصها باستخدام CRISPR
لقد أثر فيروس كورونا المستجد سلبًا على السوق. حيث تعمل عمليات الإغلاق والعزلة أثناء الأوبئة على تعقيد إدارة التشخيص والعلاج. كما أن الافتقار إلى الوصول إلى مرافق الرعاية الصحية لإدارة الرعاية الروتينية والأدوية سيؤثر بشكل أكبر على السوق. كما أن العزلة الاجتماعية تزيد من التوتر واليأس والدعم الاجتماعي، وكل هذا قد يتسبب في انخفاض الالتزام بأدوية مضادات الاختلاج أثناء الوباء.
التطورات الأخيرة
- في أغسطس 2020، أعلنت شركة SHERLOCK BIOSCIENCES عن تعاونها مع شركة Dartmouth-Hitchcock Health لإجراء التجربة السريرية لمجموعة SHERLOCK التشخيصية لفيروس Sars-CoV-2. حصلت المجموعة على موافقة طارئة من إدارة الغذاء والدواء الأمريكية (FDA) للحصول على إذن الاستخدام الطارئ.
نطاق سوق الكشف عن الجينات وتشخيصها باستخدام تقنية CRISPR في أمريكا الشمالية
يتم تقسيم سوق الكشف عن جينات CRISPR وتشخيصها على أساس ستة قطاعات: الفئة، والمنتجات والخدمات، والتطبيق، وسير العمل، والمستخدم النهائي، وقناة التوزيع. سيساعدك النمو بين هذه القطاعات على تحليل قطاعات النمو الضئيلة في الصناعات وتزويد المستخدمين بنظرة عامة قيمة على السوق ورؤى السوق لمساعدتهم على اتخاذ قرارات استراتيجية لتحديد تطبيقات السوق الأساسية.
فصل
- الفئة 1 - البروتينات متعددة التأثير
- الفئة 2 - بروتين ربط CrRNA المفرد
على أساس الفئة، يتم تقسيم سوق الكشف عن جينات CRISPR وتشخيصها إلى الفئة 1 - بروتينات متعددة التأثير والفئة 2 - بروتين ربط CrRNA الفردي.
المنتجات والخدمات
- منتجات
- خدمات
على أساس المنتجات والخدمات، يتم تقسيم سوق الكشف عن جينات CRISPR وتشخيصها إلى منتجات وخدمات.
طلب
- التشخيصات الطبية الحيوية
- هندسة الجينوم
- اكتشاف الأدوية
- التطبيقات الزراعية
- آحرون
على أساس التطبيق، يتم تقسيم سوق الكشف عن جينات CRISPR وتشخيصها إلى التشخيصات الطبية الحيوية، وهندسة الجينوم، واكتشاف الأدوية، والتطبيقات الزراعية وغيرها.
سير العمل
- إعداد العينة
- التضخيم المسبق
- الحمض النووي الريبوزي منقوص الأكسجين
- إنزيمات كاس
- الاستشعار
على أساس سير العمل، يتم تقسيم سوق الكشف عن جين CRISPR وتشخيصه إلى تحضير العينة، والتضخيم المسبق، وCrRNA، وإنزيمات Cas والاستشعار.
المستخدم النهائي
- المستشفيات
- مراكز التشخيص
- شركات التكنولوجيا الحيوية
- المؤسسات الأكاديمية والبحثية
- آحرون
على أساس المستخدم النهائي، يتم تقسيم سوق الكشف عن جينات CRISPR وتشخيصها إلى المستشفيات ومراكز التشخيص وشركات التكنولوجيا الحيوية والمعاهد الأكاديمية والبحثية وغيرها.
قناة التوزيع
- العطاءات المباشرة
- مبيعات التجزئة
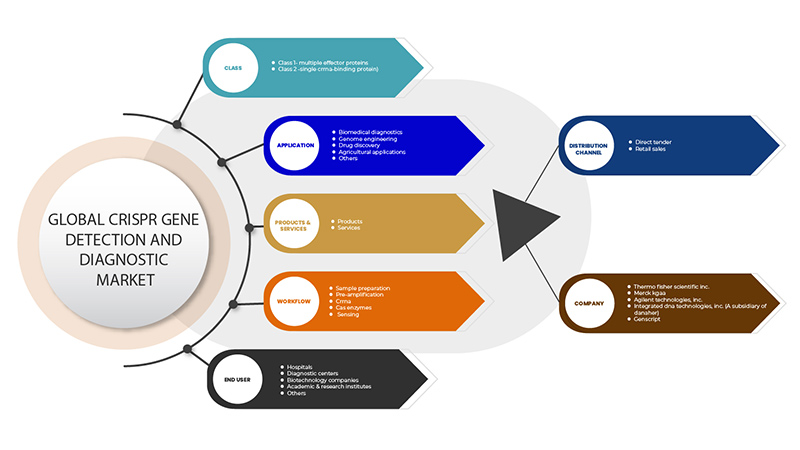
على أساس قناة التوزيع، يتم تقسيم سوق الكشف عن جين CRISPR وتشخيصه إلى العطاءات المباشرة ومبيعات التجزئة.
تحليل/رؤى إقليمية لسوق الكشف عن الجينات وتشخيصها باستخدام تقنية CRISPR
يتم تحليل سوق الكشف عن الجينات CRISPR وتشخيصها في أمريكا الشمالية وتوفير رؤى حجم السوق والاتجاهات حسب المناطق والفئة والمنتجات والخدمات والتطبيق وسير العمل والمستخدم النهائي وقناة التوزيع كما هو مذكور أعلاه.
الدول التي يغطيها تقرير سوق الكشف عن الجينات وتشخيصها باستخدام CRISPR هي الولايات المتحدة وكندا والمكسيك.
تهيمن الولايات المتحدة على سوق الكشف عن الجينات وتشخيصها باستخدام تقنية CRISPR بسبب ارتفاع الإنفاق على الرعاية الصحية.
كما يوفر قسم الدولة في التقرير عوامل التأثير الفردية على السوق والتغييرات في اللوائح في السوق محليًا والتي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. تعد نقاط البيانات مثل المبيعات الجديدة ومبيعات الاستبدال والتركيبة السكانية للدولة وعلم الأوبئة المرضية ورسوم الاستيراد والتصدير من بين المؤشرات الرئيسية المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. كما يتم النظر في وجود وتوافر العلامات التجارية في أمريكا الشمالية والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية وتأثير قنوات المبيعات أثناء تقديم تحليل تنبؤي لبيانات الدولة.
تحليل المنافسة وحصة سوق الكشف عن الجينات وتشخيصها باستخدام تقنية CRISPR
يقدم المشهد التنافسي لسوق الكشف عن جينات CRISPR وتشخيصها في أمريكا الشمالية تفاصيل حسب المنافس. تتضمن التفاصيل نظرة عامة على الشركة، والبيانات المالية للشركة، والإيرادات المولدة، وإمكانات السوق، والاستثمار في البحث والتطوير، ومبادرات السوق الجديدة، والحضور في أمريكا الشمالية، ومواقع الإنتاج والمرافق، والقدرات الإنتاجية، ونقاط القوة والضعف للشركة، وإطلاق المنتج، وعرض المنتج ونطاقه، وهيمنة التطبيق. ترتبط نقاط البيانات المذكورة أعلاه فقط بتركيز الشركات فيما يتعلق بسوق الكشف عن جينات CRISPR وتشخيصها.
بعض اللاعبين الرئيسيين العاملين في سوق الكشف عن جينات CRISPR وتشخيصها هم GenScript و OriGene Technologies، Inc. و Applied StemCell و GeneCopoeia، Inc. و Agilent Technologies، Inc. و Synthego و BioVision Inc. و Hera Biolabs و Cellecta، Inc. و New England Biolabs و 10x Genomics و addgene CasTag Biosciences و Merck KGaA و Integrated DNA Technologies، Inc. (شركة تابعة لشركة Danaher) و Thermo Fisher Scientific Inc. وغيرها.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 CLASS SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 INTELLECTUAL PROPERTY LANDSCAPE (PATENT LANDSCAPE)
6 EPIDEMIOLOGY
7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: REGULATORY SCENARIO
8 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, OF CRISPR DIAGNOSTICS
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
9.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT
9.1.3 AVAILABILITY OF FUNDING FOR CRISPR GENE DIAGNOSTICS
9.1.4 RISE IN GMP-CERTIFICATION APPROVALS FOR CRISPR GENE DIAGNOSTIC
9.1.5 RISE IN CLINICAL TRIALS FOR CRISPR BASED DIAGNOSTICS
9.2 RESTRAINTS
9.2.1 RISE IN COST OF CRISPR BASED DIAGNOSTICS
9.2.2 RISKS FACED WHILE USING CRISPR DIAGNOSIS
9.2.3 ETHICAL CONCERNS RELATED TO CRISPR GENE DETECTION AND DIAGNOSTIC RESEARCH
9.2.4 AVAILABILITY OF ALTERNATIVES
9.3 OPPORTUNITIES
9.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
9.3.2 RISE IN HEALTHCARE EXPENDITURE
9.3.3 EMERGENCE OF TECHNOLOGICAL ADVANCEMENTS IN CRISPR BASED DIAGNOSTICS
9.4 CHALLENGES
9.4.1 LACK OF SKILLED PROFESSIONALS REQUIRED FOR CRISPR DIAGNOSTICS
9.4.2 STRINGENT REGULATIONS
10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS
10.1 OVERVIEW
10.2 CLASS-2 SINGLE CRRNA-BINDING PROTEIN
10.2.1 BIOMEDICAL DIAGNOSTICS
10.2.2 AGRICULTURAL APPLICATIONS
10.2.3 GENOME ENGINEERING
10.2.4 DRUG DISCOVERY
10.2.5 OTHERS
10.3 CLASS-1 MULTIPLE EFFECTOR PROTEINS
11 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES
11.1 OVERVIEW
11.2 PRODUCTS
11.2.1 ASSAY KITS
11.2.1.1 SGRNA KIT
11.2.1.2 GENOMIC DETECTION KIT
11.2.1.3 OTHERS
11.2.2 PROTEINS
11.2.2.1 CAS9
11.2.2.2 CPF1
11.2.2.3 OTHERS
11.2.3 PLASMID AND VECTOR
11.2.4 LIBRARY
11.2.5 CONTROL KITS
11.2.6 DELIVERY SYSTEM PRODUCTS
11.2.7 DESIGN TOOLS
11.2.8 GENOMIC RNA
11.2.9 HDR BLOCKERS
11.2.9.1 AZIDOTHYMIDINE
11.2.9.2 TRIFLUOROTHYMIDINE
11.2.9.3 OTHERS
11.2.9.4 OTHERS
11.3 SERVICES
11.3.1 G-RNA DESIGN
11.3.2 CELL LINE ENGINEERING
11.3.3 MICROBIAL GENE EDITING
11.3.4 DNA SYNTHESIS
11.3.5 OTHERS
12 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 BIOMEDICAL DIAGNOSTICS
12.2.1 CANCER
12.2.2 BLOOD DISORDERS
12.2.3 HEREDITARY DISORDERS
12.2.4 MUSCULAR DYSTROPHY
12.2.5 AIDS
12.2.6 NEURODEGENERATIVE CONDITION
12.2.7 OTHERS
12.3 AGRICULTURAL APPLICATIONS
12.4 GENOME ENGINEERING
12.4.1 CELL LINE ENGINEERING
12.4.2 HUMAN STEM CELLS
12.5 DRUG DISCOVERY
12.6 OTHERS
13 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW
13.1 OVERVIEW
13.2 CRRNA
13.3 CAS ENZYME
13.4 PRE-AMPLIFICATION
13.4.1 PCR
13.4.2 LAMP
13.4.3 RPA
13.5 SAMPLE PREPARATION
13.6 SENSING
13.6.1 FLUORESCENT PROBES
13.6.2 COLORIMETRIC
14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER
14.1 OVERVIEW
14.2 BIOTECHNOLOGY COMPANIES
14.3 ACADEMIC AND RESEARCH INSTITUTES
14.4 DIAGNOSTIC CENTERS
14.5 HOSPITALS
14.6 OTHERS
15 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
16 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 THERMO FISHER SCIENTIFIC INC.
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENTS
19.2 MERCK KGA
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 AGILENT TECHNILOGIES, INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 INTEGRATED DNA TECHNOLOGIES, INC. (A SUBSIDIARY OF DANAHER)
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 GENSCRIPT
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 10 X GENOMICS
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.7 APPLIED STEM CELL
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 ADDGENE
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 BIOVISION INC.
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CELLECTA, INC
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENTS
19.11 CAS TAG BIOSCIENCES
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 GENECOPOEIA, INC.
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 HORIZON DISCOVERY LTD
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENTS
19.14 HERA BIOLABS
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 NEW ENGLAND BIOLABS
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENTS
19.16 ORIGENE TECHNOLOGIES, INC.
19.16.1 COMPANY SNAPSHOT
19.16.2 PRODUCT PORTFOLIO
19.16.3 RECENT DEVELOPMENT
19.17 SYNTHEGO
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENTS
19.18 TAKARA BIO INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 TOOLGEN, INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 PRODUCT PORTFOLIO
19.19.3 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
List of Table
TABLE 1 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE THERAPEUTICS
TABLE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA CLASS-1 MULTIPLE EFFECTOR PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA AGRICULTURAL APPLICATIONS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA DRUG DISCOVERYIN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA CRRNA IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA CAS ENZYME IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SAMPLE PREPARATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA ACADEMIC AND RESEARCH INSTITUTES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA DIAGNOSTIC CENTERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA DIRECT TENDER IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL SALES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 57 U.S. CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 58 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 59 U.S. PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 U.S. ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 62 U.S. PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 63 U.S. SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 65 U.S. GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 U.S. BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 68 U.S. PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 69 U.S. SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 70 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 72 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 73 CANADA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 75 CANADA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 CANADA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 77 CANADA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 CANADA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 80 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 CANADA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 82 CANADA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 84 CANADA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 85 CANADA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 86 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 88 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 89 MEXICO CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 90 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 91 MEXICO PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 92 MEXICO ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 93 MEXICO HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 94 MEXICO PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 95 MEXICO SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 96 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 98 MEXICO BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 99 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 100 MEXICO PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 101 MEXICO SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DBMR POSITION GRID
FIGURE 8 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN TECHNOLOGICAL ADVANCEMENTS IN CRISPR DIAGNOSTICS, AND GOVERNMENT FUNDING FOR THE DEVELOPMENT OF CRISPR DETECTION KITS ARE EXPECTED TO DRIVE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 TO 2029
FIGURE 13 CLASS SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 & 2029
FIGURE 14 NORTH AMERICA CRISPR GENE PATENT SCENARIO, BY APPLICATION
FIGURE 15 CRISPR PATENT LANDSCAPE AND NUMBER OF APPLICATIONS OF NEW PATENT FAMILIES FILED WORLDWIDE, 2001 TO 2019
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
FIGURE 17 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 18 PREVALENCE OF HUNTINGTON’S DISEASE IN 2019
FIGURE 19 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2021
FIGURE 20 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2022-2029 (USD MILLION)
FIGURE 21 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, CAGR (2022-2029)
FIGURE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, LIFELINE CURVE
FIGURE 23 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2021
FIGURE 24 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2022-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, LIFELINE CURVE
FIGURE 27 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2021
FIGURE 28 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 29 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 31 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2021
FIGURE 32 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2022-2029 (USD MILLION)
FIGURE 33 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, LIFELINE CURVE
FIGURE 35 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 44 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 45 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS (2022-2029)
FIGURE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.