سوق غرسات الوجه والفكين في أمريكا الشمالية، حسب النوع (بديل الطعم العظمي، غرسات منتصف الوجه، غرسات الجمجمة/الأعصاب، غرسات تقويم الفك السفلي، نظام التشتيت، أنظمة تثبيت رفرف الجمجمة، غرسات خاصة بالمريض (PSI)، نظام استبدال المفصل الصدغي الفكي الكلي (TMJ)، منتجات إصلاح الجافية، أخرى)، مادة البناء (المعدن، بديل الطعم العظمي، البوليمرات/المواد الحيوية، أخرى)، موقع التطبيق (المثبتات الداخلية، المثبتات الخارجية)، نوع الجراحة ( جراحة إعادة البناء ، جراحات الصدمات، جراحات التجميل ، جراحات تقويم الفكين، جراحات الأسنان، جراحات الأنف والأذن والحنجرة، أخرى)، نوع الملكية (المثبتات غير القابلة للامتصاص، المثبتات القابلة للامتصاص)، المستخدم النهائي (المستشفيات، العيادات المتخصصة، مراكز الصدمات، مراكز الجراحة الخارجية (ASCs)، أخرى)، قناة التوزيع (المباشرة اتجاهات الصناعة وتوقعاتها حتى عام 2029 (العطاءات، مبيعات التجزئة، الدولة (الولايات المتحدة، كندا، المكسيك)).
تحليل السوق والرؤى : سوق زراعة القحف والفكين في أمريكا الشمالية
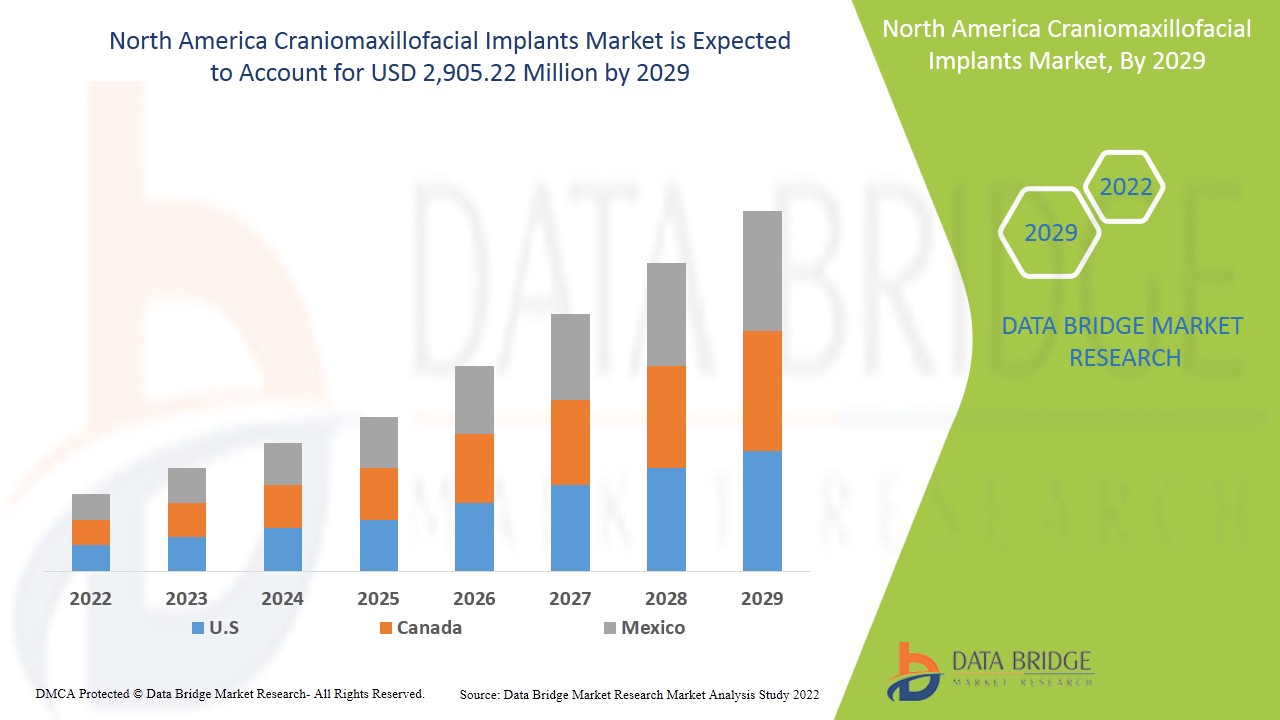
من المتوقع أن يحقق سوق زراعة القحف والفك والوجه في أمريكا الشمالية نموًا في السوق في الفترة المتوقعة من 2022 إلى 2029. تحلل شركة Data Bridge Market Research أن السوق ينمو بمعدل نمو سنوي مركب بنسبة 8.0٪ في الفترة المتوقعة من 2022 إلى 2029 ومن المتوقع أن يصل إلى 2،905.22 مليون دولار أمريكي بحلول عام 2029. التقدم التكنولوجي المتزايد هو المحرك الرئيسي الذي دفع الطلب على السوق في الفترة المتوقعة.
تُستخدم الغرسات القحفية الفكية الوجهية في جراحات الوجه والفكين. تحدث التشوهات القحفية الفكية الوجهية أثناء الولادة بسبب نقص حمض الفوليك لدى الأم أو الطفرات الجينية. كما تتسبب عيوب الوجه في إتلاف الكلام والأكل واللغة وتسبب مشاكل الأسنان وفقدان السمع. يتم إجراؤها عمومًا لعلاج الإصابات والعيوب والأمراض في الرأس والرقبة والوجه والفكين والأنسجة الصلبة واللينة في منطقة الفم والوجه والفكين.
إن الزيادة في عدد السكان المسنين وارتفاع معدل انتشار سرطان الأمعاء والمثانة يعملان على تسريع نمو السوق. وقد حفز تكييف التقنيات المتقدمة في الصناعة نمو السوق وبالتالي، من المرجح أن يؤدي الطلب المتزايد على الغرسات المخصصة للمرضى في العمليات الجراحية إلى دفع نمو السوق في الفترة المتوقعة.
يقدم تقرير سوق غرسات الوجه والفكين تفاصيل عن حصة السوق والتطورات الجديدة وتحليل خط أنابيب المنتجات وتأثير اللاعبين المحليين والمحليين في السوق وتحليل الفرص من حيث جيوب الإيرادات الناشئة والتغييرات في لوائح السوق وموافقات المنتجات والقرارات الاستراتيجية وإطلاق المنتجات والتوسعات الجغرافية والابتكارات التكنولوجية في السوق. لفهم التحليل وسيناريو السوق، اتصل بنا للحصول على موجز محلل، وسيساعدك فريقنا في إنشاء حل لتأثير الإيرادات لتحقيق هدفك المنشود.
نطاق وحجم سوق زراعة القحف والفك والوجه في أمريكا الشمالية
يتم تقسيم سوق زراعة القحف والفكين على أساس النوع ومواد البناء وموقع التطبيق ونوع الجراحة ونوع الملكية والمستخدم النهائي وقناة التوزيع.
- على أساس النوع، يتم تقسيم سوق غرسات الوجه والفكين إلى بدائل طعوم العظام، وغرسات منتصف الوجه، وغرسات الجمجمة/الأعصاب، وغرسات تقويم الفك السفلي، ونظام التشتيت، وأنظمة تثبيت رفرف الجمجمة، وغرسات خاصة بالمريض (PSI)، ونظام استبدال المفصل الصدغي الفكي الكلي (TMJ)، ومنتجات إصلاح الجافية، وغيرها. في عام 2022، من المتوقع أن تهيمن شريحة بدائل طعوم العظام على سوق غرسات الوجه والفكين العالمية بسبب زيادة عدد السكان المسنين على مستوى العالم.
- على أساس مادة البناء، يتم تقسيم سوق غرسات الوجه والفكين إلى المعدن، وبديل ترقيع العظام، والبوليمرات/المواد الحيوية، وغيرها. في عام 2022، من المتوقع أن تهيمن المعادن على سوق غرسات الوجه والفكين العالمية مع التقدم التكنولوجي المتزايد ونفقات الرعاية الصحية الحكومية.
- على أساس موقع التطبيق، يتم تقسيم سوق غرسات الوجه والفكين إلى مثبتات خارجية ومثبتات داخلية. في عام 2022، من المتوقع أن تهيمن المثبتات الخارجية على سوق غرسات الوجه والفكين العالمية مع زيادة إطلاق المنتجات والاستحواذ عليها.
- على أساس نوع الجراحة، يتم تقسيم سوق غرسات الوجه والفكين إلى جراحة إعادة البناء، وجراحات الصدمات، والجراحات التجميلية، وجراحات تقويم الفكين، وجراحات الأسنان، وجراحات الأنف والأذن والحنجرة، وغيرها. في عام 2022، من المتوقع أن تهيمن جراحات إعادة البناء على سوق غرسات الوجه والفكين العالمية مع زيادة عدد العمليات الجراحية والإجراءات.
- على أساس نوع الملكية، يتم تقسيم سوق غرسات الوجه والفكين إلى مثبتات قابلة للامتصاص ومثبتات غير قابلة للامتصاص. في عام 2022، من المتوقع أن تهيمن المثبتات القابلة للامتصاص على سوق غرسات الوجه والفكين العالمية لأنها توفر نتيجة أفضل بمضاعفات أقل.
- على أساس المستخدم النهائي، يتم تقسيم سوق غرسات الوجه والفكين إلى المستشفيات والعيادات المتخصصة ومراكز الصدمات ومراكز الجراحة الخارجية وغيرها. في عام 2022، من المتوقع أن تهيمن المستشفيات على سوق غرسات الوجه والفكين العالمية مع زيادة عدد المرضى والعمليات الجراحية.
- على أساس قناة التوزيع، يتم تقسيم سوق غرسات الوجه والفكين إلى عطاءات مباشرة ومبيعات التجزئة. في عام 2022، من المتوقع أن تهيمن العطاءات المباشرة على سوق غرسات الوجه والفكين العالمية حيث تمتلك جميع المنتجات الرئيسية من كبار اللاعبين في السوق.
تحليل سوق زراعة القحف والفكين على مستوى الدولة
يتم تقسيم سوق زراعة القحف والفكين على أساس النوع ومواد البناء وموقع التطبيق ونوع الجراحة ونوع الملكية والمستخدم النهائي وقناة التوزيع.
الدول التي يغطيها تقرير سوق زراعة الجمجمة والوجه والفكين هي الولايات المتحدة وكندا والمكسيك.
- وفي أمريكا الشمالية، من المتوقع أن تهيمن الولايات المتحدة على السوق بسبب وجود لاعبين رئيسيين في السوق.
كما يوفر قسم الدولة في التقرير عوامل التأثير الفردية على السوق والتغييرات في التنظيم في السوق محليًا والتي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. تعد نقاط البيانات مثل المبيعات الجديدة ومبيعات الاستبدال والتركيبة السكانية للدولة والقوانين التنظيمية ورسوم الاستيراد والتصدير من بين المؤشرات الرئيسية المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. كما يتم النظر في وجود وتوافر العلامات التجارية العالمية والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية وتأثير قنوات المبيعات أثناء تقديم تحليل توقعات لبيانات الدولة.
زيادة تمويل القطاع الخاص والتقدم التكنولوجي يعززان نمو سوق زراعة الأسنان في الوجه والفكين
يوفر لك سوق زراعة الأسنان في الوجه والفكين أيضًا تحليلًا تفصيليًا للسوق لكل دولة من حيث نمو صناعة زراعة الأسنان مع تأثير التقدم والتكنولوجيا والتغييرات في السيناريوهات التنظيمية. تتوفر البيانات للفترة التاريخية من 2011 إلى 2019.
تحليل المنافسة وحصة سوق غرسات الوجه والفكين
يوفر المشهد التنافسي لسوق زراعة الوجه والفكين تفاصيل حسب المنافس. تتضمن التفاصيل نظرة عامة على الشركة، والبيانات المالية للشركة، والإيرادات المتولدة، وإمكانات السوق، والاستثمار في البحث والتطوير، ومبادرات السوق الجديدة، ومواقع الإنتاج والمرافق، ونقاط القوة والضعف في الشركة، وإطلاق المنتج، وخطوط أنابيب تجارب المنتج، وموافقات المنتج، وبراءات الاختراع، وعرض المنتج والتنفس، وهيمنة التطبيق، ومنحنى شريان الحياة التكنولوجي. ترتبط نقاط البيانات المذكورة أعلاه فقط بتركيز الشركة فيما يتعلق بسوق زراعة الوجه والفكين.
الشركات الكبرى التي تتعامل في سوق زراعة الوجه والفكين هي Zimmer Biomet و Stryker و Johnson and Johnson Services Inc. و Medtronic و KLS Martin Group و Delphos Implants و Osteomed و Anatomics Pty Ltd و Bioplate Inc. و Calavera Surgical Design و Innovasis و Integra LifeSciences Holdings Corp. و Dimeda Instrumente GmbH و General Implants GmbH و Jeil Medical Corporation و Xilloc Medical BV و Ortho Select GmbH و B. Braun Melsungen AG و MONDEAL Medical Systems GmbH و OssDsign AB و TREU-Instrumente GmbH و Rebstock Instruments GmbH و 3D Systems، Inc. و Medartis AG و BIOPORE SURGICALS و AlloSource و Teknimed و Poriferous و 7s Medical AG و Innovation Medical GmbH و Advin Health care و Gesco Healthcare Pvt. المحدودة، شركة أوكسين الطبية الخاصة المحدودة رينيشو بي إل سي، شركة أورثو ماكس للتصنيع الخاصة المحدودة، فاست أورثو، شركة سكول إمبلانتس، بانثيرا دينتال، لوسيد إمبلانتس وغيرها.
- في أكتوبر 2021، أعلنت AlloSource أنها ستطلق AlloMend® Extra-Large (XL) Acellular Dermal Matrix (ADM)، وهو أحدث إضافة إلى خط منتجات AlloMend. وقد أدى هذا إلى زيادة خط إنتاج الشركة.
- في أغسطس 2021، أعلنت شركة Medtronic plc عن اتفاقيتها مع Intersect ENT، الشركة العالمية الرائدة في مجال التكنولوجيا الطبية في مجال الأذن والأنف والحنجرة. يعمل استحواذ Medtronic على Intersect ENT على توسيع محفظة الشركة من المنتجات المستخدمة أثناء إجراءات الأذن والأنف والحنجرة.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES MODEL
5 INDUSTRIAL INSIGHTS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE RATE OF CONGENITAL FACIAL DEFORMITIES
6.1.2 INCREASE IN TRAUMA CASES AND ROAD ACCIDENTS
6.1.3 INCREASING DEMAND FOR FACIAL RECONSTRUCTION SURGERY
6.1.4 RECENT TECHNOLOGICAL ADVANCEMENTS IN CMF IMPLANTS
6.2 RESTRAINS
6.2.1 HIGH COST OF SURGERIES AND IMPLANTS
6.2.2 IMPLANT MALFUNCTION AND ASSOCIATED RISK
6.2.3 LACK OF AWARENESS IN DEVELOPING COUNTRIES
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISE IN HEALTHCARE EXPENDITURE & INFRASTRUCTURE
6.3.3 GROWING R&D ACTIVITIES AND TECHNOLOGICAL ADVANCEMENT
6.3.4 INCREASE IN DEMAND FOR CUSTOMIZED AND PATIENT-SPECIFIC IMPLANTS
6.4 CHALLENGE
6.4.1 INCREASE IN PRODUCT RECALL
6.4.2 POST SURGERY COMPLIACTION
7 COVID-19 IMPACT ON NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
7.1 PRICE IMPACT
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY
7.4 STRATEGIC DECISIONS BY MANUFACTURERS
7.5 CONCLUSION
8 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE
8.1 OVERVIEW
8.2 BONE GRAFT SUBSTITUTE
8.2.1 NATURAL
8.2.2 SYNTHETIC
8.3 MID-FACE IMPLANTS
8.3.1 PLATES
8.3.2 SCREWS
8.4 CRANIAL/NEURO IMPLANTS
8.4.1 PLATES
8.4.2 SCREWS
8.4.3 CONTOURABLE MESHES
8.5 MANDIBULAR ORTHOGNATHIC IMPLANTS
8.5.1 PLATES
8.5.2 SCREWS
8.6 DISTRACTION SYSTEM
8.7 CRANIAL FLAP FIXATION SYSTEMS
8.8 PATIENT SPECIFIC IMPLANTS (PSI)
8.8.1 METAL
8.8.2 PLASTIC
8.8.3 OTHERS
8.8.3.1 NORMAL
8.8.3.2 3D-PRINTED
8.8.3.2.1 CAD
8.8.3.2.2 ADEPT SOFTWARE
8.8.3.2.3 CAM
8.8.3.2.4 OTHERS
8.9 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
8.1 DURAL REPAIR PRODUCTS
8.10.1 DURAL SEALANTS
8.10.2 DURAL SUBSTITUTES
8.11 OTHERS
9 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION
9.1 OVERVIEW
9.2 METALS
9.2.1 TITANIUM AND ALLOYS
9.2.2 STAINLESS STEEL
9.2.3 POLYETHERETHERKETONE (PEEK)
9.2.4 SILICON NITRIDE
9.2.5 OTHERS
9.3 BONE GRAFT SUBSTITUTE
9.4 POLYMERS/BIOMATERIAL
9.5 OTHERS
10 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE
10.1 OVERVIEW
10.2 INTERNAL FIXATOR
10.2.1 BONE GRAFT SUBSTITUTE
10.2.2 MID-FACE IMPLANTS
10.2.3 CRANIAL/NEURO IMPLANTS
10.2.4 MANDIBULAR ORTHOGNATHIC
10.2.5 DISTRACTION SYSTEM
10.2.6 CRANIAL FLAP FIXATION SYSTEMS
10.2.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
10.2.8 OTHERS
10.3 EXTERNAL FIXATOR
10.3.1 DISTRACTION SYSTEM
10.3.2 OTHERS
11 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE
11.1 OVERVIEW
11.2 RECONSTRUCTIVE SURGERY
11.2.1 BONE GRAFT SUBSTITUTE
11.2.2 MID-FACE IMPLANTS
11.2.3 CRANIAL/NEURO IMPLANTS
11.2.4 MANDIBULAR ORTHOGNATHIC
11.2.5 DISTRACTION SYSTEM
11.2.6 CRANIAL FLAP FIXATION SYSTEMS
11.2.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.2.8 DURAL REPAIR
11.2.9 OTHERS
11.3 TRAUMA SURGERY
11.3.1 BONE GRAFT SUBSTITUTE
11.3.2 MID-FACE IMPLANTS
11.3.3 CRANIAL/NEURO IMPLANTS
11.3.4 MANDIBULAR ORTHOGNATHIC
11.3.5 DISTRACTION SYSTEM
11.3.6 CRANIAL FLAP FIXATION SYSTEMS
11.3.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.3.8 DURAL REPAIR
11.3.9 OTHERS
11.4 PLASTIC SURGERIES
11.4.1 MID-FACE IMPLANTS
11.4.2 MANDIBULAR ORTHOGNATHIC
11.4.3 CRANIAL/NEURO IMPLANTS
11.4.4 DISTRACTION SYSTEM
11.4.5 CRANIAL FLAP FIXATION SYSTEMS
11.4.6 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.4.7 OTHERS
11.5 ORTHOGNATHIC SURGERIES
11.5.1 BONE GRAFT SUBSTITUTE
11.5.2 MANDIBULAR ORTHOGNATHIC
11.5.3 DISTRACTION SYSTEM
11.5.4 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.5.5 OTHERS
11.6 DENTAL SURGERIES
11.6.1 DISTRACTION SYSTEM
11.6.2 OTHERS
11.7 ENT SURGERIES
11.7.1 BONE GRAFT SUBSTITUTE
11.7.2 MID-FACE IMPLANTS
11.7.3 OTHERS
11.8 OTHERS
12 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE
12.1 OVERVIEW
12.2 NON-RESORBABLE FIXATORS
12.3 RESORBABLE FIXATORS
13 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CLINICS
13.4 TRAUMA CENTERS
13.5 AMBULATORY SURGICAL CENTERS (ASCS)
13.6 OTHERS
14 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
15 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 ZIMMER BIOMET.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 RODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 MEDTRONIC
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENT
18.3 DEPUY SYNTHES.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 STRYKER
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 ALLOSOURCE
18.5.1 COMPANY SNAPSHOT
18.5.2 COMPANY SHARE ANALYSIS
18.5.3 PRODUCT PORTFOLIO
18.5.4 RECENT DEVELOPMENT
18.5.4.1 FDA APPROVAL
18.6 ADVIN HEALTH CARE
18.6.1 COMPANY SNAPSHOT
18.6.2 PRODUCT PORTFOLIO
18.6.3 RECENT DEVELOPMENT
18.7 AESCULAP AG
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENT
18.8 ANATOMICS PTY LTD.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 AUXEIN MEDICAL PRIVATE LIMITED
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENT
18.1 BIOPLATE INC.
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENT
18.11 BIOPORE SURGICALS
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENT
18.12 CALAVERA SURGICAL DESIGN
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENT
18.13 DELPHOS IMPLANTS.
18.13.1 COMPANY SNAPSHOT
18.13.2 PRODUCT PORTFOLIO
18.13.3 RECENT DEVELOPMENT
18.14 DIMEDA INSTRUMENTE GMBH
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENTS
18.15 GENERAL-IMPLANTS GMBH
18.15.1 COMPANY SNAPSHOT
18.15.2 PRODUCT PORTFOLIO
18.15.3 RECENT DEVELOPMENT
18.16 GESCO HEALTHCARE PVT. LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 INNOVATION MEDICAL GMBH
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENT
18.17.3.1 MANUFACTURING CERTIFICATION
18.18 INNOVASIS INC.
18.18.1 COMPANY SNAPSHOT
18.18.2 PRODUCT PORTFOLIO
18.18.3 RECENT DEVELOPMENT
18.19 JEIL MEDICAL CORPORATION
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 KLS-MARTIN L.P.
18.20.1 COMPANY SNAPSHOT
18.20.2 PRODUCT PORTFOLIO
18.20.3 RECENT DEVELOPMENT
18.21 LUCID IMPLANTS
18.21.1 COMPANY SNAPSHOT
18.21.2 PRODUCT PORTFOLIO
18.21.3 RECENT DEVELOPMENT
18.22 MEDARTIS AG
18.22.1 COMPANY SNAPSHOT
18.22.2 PRODUCT PORTFOLIO
18.22.3 RECENT DEVELOPMENT
18.23 MONDEAL MEDICAL SYSTEMS GMBH
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 ORTHO MAX MANUFACTURING COMPANY PVT. LTD.
18.24.1 COMPANY SNAPSHOT
18.24.2 PRODUCT PORTFOLIO
18.24.3 RECENT DEVELOPMENT
18.25 ORTHO SELECT GMBH
18.25.1 COMPANY SNAPSHOT
18.25.2 PRODUCT PORTFOLIO
18.25.3 RECENT DEVELOPMENT
18.26 OSTEOMED
18.26.1 COMPANY SNAPSHOT
18.26.2 PRODUCT PORTFOLIO
18.26.3 RECENT DEVELOPMENT
18.27 OSSDSIGN AB
18.27.1 COMPANY SNAPSHOT
18.27.2 PRODUCT PORTFOLIO
18.27.3 RECENT DEVELOPMENT
18.27.3.1 PRODUCT LAUNCH
18.27.3.2 ACQUISITION
18.28 PANTHERA DENTAL
18.28.1 COMPANY SNAPSHOT
18.28.2 PRODUCT PORTFOLIO
18.28.3 RECENT DEVELOPMENT
18.29 PORIFEROUS
18.29.1 COMPANY SNAPSHOT
18.29.2 PRODUCT PORTFOLIO
18.29.3 RECENT DEVELOPMENT
18.29.3.1 MANUFACTURING CERTIFICATION
18.3 REBSTOCK INSTRUMENTS GMBH
18.30.1 COMPANY SNAPSHOT
18.30.2 PRODUCT PORTFOLIO
18.30.3 RECENT DEVELOPMENT
18.31 RENISHAW PLC
18.31.1 COMPANY SNAPSHOT
18.31.2 REVENUE ANALYSIS
18.31.3 PRODUCT PORTFOLIO
18.31.4 RECENT DEVELOPMENT
18.32 SKULLE IMPLANTS CORPORATION.
18.32.1 COMPANY SNAPSHOT
18.32.2 PRODUCT PORTFOLIO
18.32.3 RECENT DEVELOPMENT
18.33 TEKNIMED
18.33.1 COMPANY SNAPSHOT
18.33.2 PRODUCT PORTFOLIO
18.33.3 RECENT DEVELOPMENT
18.33.3.1 MDSAP CERTIFICATION
18.34 TREU-INSTRUMENTE GMBH
18.34.1 COMPANY SNAPSHOT
18.34.2 PRODUCT PORTFOLIO
18.34.3 RECENT DEVELOPMENT
18.35 VAST ORTHO: ORTHOPEDIC IMPLANTS MANUFACTURERS
18.35.1 COMPANY SNAPSHOT
18.35.2 PRODUCT PORTFOLIO
18.35.3 RECENT DEVELOPMENTS
18.36 XILLOC MEDICAL BV
18.36.1 COMPANY SNAPSHOT
18.36.2 PRODUCT PORTFOLIO
18.36.3 RECENT DEVELOPMENT
18.37 7S MEDICAL AG
18.37.1 COMPANY SNAPSHOT
18.37.2 PRODUCT PORTFOLIO
18.37.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
List of Table
TABLE 1 UNITS/NUMBER OF PROCEDURES PERFORMED PER YEAR
TABLE 2 PRICING FOR VARIOUS PROCEDURES
TABLE 3 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 4 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 5 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 6 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 7 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 8 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 9 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 10 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 11 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 12 NORTH AMERICA DISTRACTION SYSTEM IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CRANIAL FLAP FIXATION SYSTEMS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 14 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 15 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 16 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 17 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 18 NORTH AMERICA TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 19 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 20 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 21 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 23 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 24 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 25 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 26 NORTH AMERICA POLYMERS/BIOMATERIAL IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 27 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 28 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 29 NORTH AMERICA INTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 30 NORTH AMERICA INTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 31 NORTH AMERICA EXTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 32 NORTH AMERICA EXTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 33 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 34 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 35 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 36 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 37 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 38 NORTH AMERICA PLASTIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 39 NORTH AMERICA PLASTIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 40 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 41 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 42 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 43 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 44 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 45 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 46 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 47 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 48 NORTH AMERICA NON-RESORBABLE FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 49 NORTH AMERICA RESORBABLE FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 50 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 51 NORTH AMERICA HOSPITAL IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 52 NORTH AMERICA SPECIALTY CLINICS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 53 NORTH AMERICA TRAUMA CENTERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 54 NORTH AMERICA AMBULATORY SURGICAL CENTERS (ASCS) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 55 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 56 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 57 NORTH AMERICA DIRECT TENDER IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 58 NORTH AMERICA RETAIL SALES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 59 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY COUNTRY, 2015-2029 (USD MILLION)
TABLE 60 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 61 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 62 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 63 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 64 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 65 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 66 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 67 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 68 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 69 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 70 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 71 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 72 NORTH AMERICA INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 73 NORTH AMERICA EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 74 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 75 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 76 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 77 NORTH AMERICA PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 78 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 79 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 80 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 81 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 82 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 83 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 84 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 85 U.S. BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 86 U.S. MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 87 U.S. CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 88 U.S. MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 89 U.S. DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 90 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 91 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 92 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 93 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 94 U.S. METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 95 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 96 U.S. INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 97 U.S. EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 98 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 99 U.S. RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 100 U.S. TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 101 U.S. PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 102 U.S. ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 103 U.S. DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 104 U.S. ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 105 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 106 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 107 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 108 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 109 CANADA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 110 CANADA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 111 CANADA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 112 CANADA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 113 CANADA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 114 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 115 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 116 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 117 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 118 CANADA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 119 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 120 CANADA INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 121 CANADA EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 122 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 123 CANADA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 124 CANADA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 125 CANADA PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 126 CANADA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 127 CANADA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 128 CANADA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 129 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 130 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 131 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 132 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 133 MEXICO BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 134 MEXICO MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 135 MEXICO CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 136 MEXICO MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 137 MEXICO DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 138 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 139 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 140 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 141 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 142 MEXICO METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 143 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 144 MEXICO INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 145 MEXICO EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 146 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 147 MEXICO RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 148 MEXICO TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 149 MEXICO PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 150 MEXICO ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 151 MEXICO DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 152 MEXICO ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 153 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 154 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 155 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: NORTH AMERICA VS COUNTRY MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SEGMENTATION
FIGURE 11 INCREASE IN DEMAND FOR PATIENTS SPECIFIC IMPLANTS ARE EXPECTED TO DRIVE THE NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 BONE GRAFT SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET IN 2022 & 2029
FIGURE 13 DRIVER, RESTRAINS, OPPORTUNITIES & CHALLENGES OF NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
FIGURE 14 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, 2021
FIGURE 15 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, 2020-2029 (USD MILLION)
FIGURE 16 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 17 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, 2021
FIGURE 19 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, 2020-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, LIFELINE CURVE
FIGURE 22 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, 2021
FIGURE 23 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, 2020-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, 2021
FIGURE 27 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, 2020-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, 2021
FIGURE 31 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, 2020-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 39 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 40 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 41 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SNAPSHOT (2021)
FIGURE 43 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2021)
FIGURE 44 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 45 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 46 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE (2022-2029)
FIGURE 47 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY SHARE 2021 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.