سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا، حسب النوع (اختبار الناقل، الاختبار التشخيصي، فحص حديثي الولادة، الاختبار التنبؤي والأعراضي، الاختبار قبل الولادة، أنواع أخرى)، التكنولوجيا (تسلسل الحمض النووي (الاختبار القائم على NGS)، تفاعل البوليميراز المتسلسل، المصفوفات الدقيقة، تسلسل الجينوم الكامل، التهجين الموضعي الفلوري (FISH)، وغيرها)، الأمراض (اضطراب وراثي نادر، السرطان، التليف الكيسي ، الاختبارات الجينية التناسلية، الاختبارات الجينية للصحة والعافية، فقر الدم المنجلي، ضمور العضلات دوشين، الثلاسيميا، مرض هنتنغتون، متلازمة X الهش، وغيرها)، المستخدم النهائي (المستشفيات والعيادات ومراكز التشخيص والعيادات الخاصة ومقدمي خدمات المختبرات والمختبرات الخاصة) اتجاهات الصناعة والتوقعات حتى عام 2030.
تحليل ورؤى حول سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
إن سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا مدفوع بعوامل مثل ارتفاع معدل انتشار الاضطرابات الوراثية، والتقدم التكنولوجي المتزايد في سوق الاختبارات الجينية، مما يعزز الطلب عليها، فضلاً عن زيادة الاستثمار في البحث والتطوير، مما يؤدي إلى نمو السوق. في الوقت الحالي، زادت نفقات الرعاية الصحية في البلدان المتقدمة والناشئة، ومن المتوقع أن يخلق ذلك ميزة تنافسية للشركات المصنعة لتطوير أسواق جديدة ومبتكرة للاختبارات الجينية.
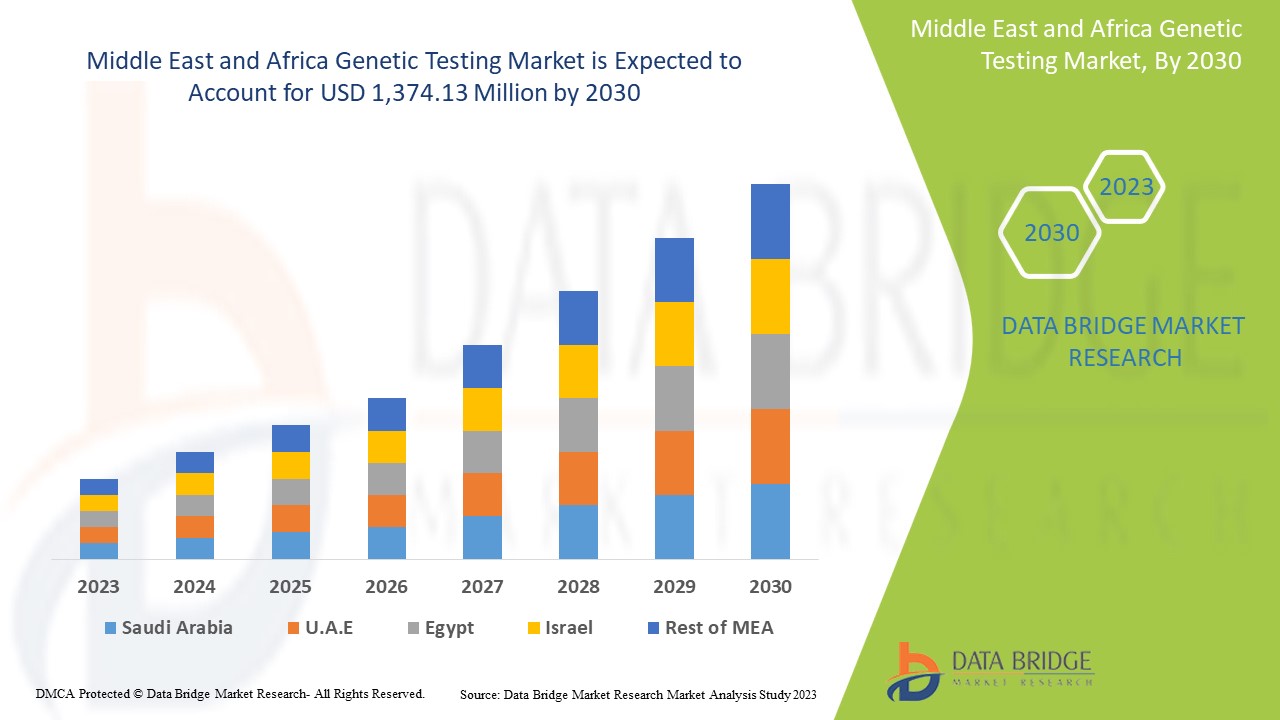
من المتوقع أن يحقق سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا نموًا في السوق في الفترة المتوقعة من 2023 إلى 2030. تحلل شركة Data Bridge Market Research أن السوق ينمو بمعدل نمو سنوي مركب قدره 13.6٪ في الفترة المتوقعة من 2023 إلى 2030 ومن المتوقع أن يصل إلى 1،374.13 مليون دولار أمريكي بحلول عام 2030.
يقدم تقرير سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا تفاصيل عن حصة السوق والتطورات الجديدة وتحليل خط أنابيب المنتجات وتأثير اللاعبين المحليين والمحليين في السوق وتحليل الفرص من حيث جيوب الإيرادات الناشئة والتغييرات في لوائح السوق وموافقات المنتجات والقرارات الاستراتيجية وإطلاق المنتجات والتوسعات الجغرافية والابتكارات التكنولوجية في السوق. لفهم التحليل وسيناريو السوق، اتصل بنا للحصول على موجز محلل، وسيساعدك فريقنا في إنشاء حل تأثير الإيرادات لتحقيق هدفك المنشود.
|
تقرير القياس |
تفاصيل |
|
فترة التنبؤ |
2023 إلى 2030 |
|
سنة الأساس |
2022 |
|
سنوات تاريخية |
2021 (قابلة للتخصيص حتى 2020-2015) |
|
وحدات كمية |
الإيرادات بالملايين من الدولارات الأمريكية |
|
القطاعات المغطاة |
حسب النوع (اختبار الناقل، الاختبار التشخيصي، فحص حديثي الولادة، الاختبار التنبؤي والاختبار ما قبل الأعراض، الاختبار قبل الولادة، أنواع أخرى)، التكنولوجيا (تسلسل الحمض النووي (الاختبار القائم على NGS)، تفاعل البوليميراز المتسلسل، المصفوفات الدقيقة، تسلسل الجينوم الكامل، التهجين الموضعي الفلوري (FISH)، وغيرها)، الأمراض (اضطراب وراثي نادر، السرطان، التليف الكيسي، الاختبار الجيني التناسلي، الاختبار الجيني للصحة والعافية، فقر الدم المنجلي، ضمور العضلات دوشين، الثلاسيميا، مرض هنتنغتون، متلازمة كروموسوم إكس الهش، وغيرها)، المستخدم النهائي (المستشفيات والعيادات ومراكز التشخيص والعيادات الخاصة ومقدمي خدمات المختبرات والمختبرات الخاصة). |
|
الدول المغطاة |
جنوب أفريقيا، المملكة العربية السعودية، الإمارات العربية المتحدة، مصر، إسرائيل، وبقية دول الشرق الأوسط وأفريقيا. |
|
الجهات الفاعلة في السوق المشمولة |
Abbott، و23ANDME، Inc.، وDanaher، وMyriad Genetics، Inc.، وOCP Medical Centre، LLC، وHealthchecks360، وBayer AG، وBioReference Health، LLC (شركة تابعة لشركة OPKO Health، Inc.)، وFREIBURG MEDICAL LABORATORY MIDDLE EAST (LLC)، وThermo Fisher Scientific Inc.، وBio-Rad Laboratories، Inc.، وBiocartis، وEurofins Scientific، وPerkinElmer Inc.، وELITechGroup، وF. Hoffmann-La Roche Ltd.، وIllumina، Inc.، وQIAGEN، وBIO-HELIX، وPacBio وغيرها. |
تعريف السوق
الاختبار الجيني هو نوع من الاختبارات الطبية التي تحدد التغيرات في الجينات أو الكروموسومات أو البروتينات. يمكن لنتيجة الاختبار الجيني تأكيد أو استبعاد حالة وراثية مشتبه بها أو المساعدة في تحديد فرصة الشخص في الإصابة باضطراب وراثي أو نقله. هناك أكثر من 77000 اختبار جيني قيد الاستخدام حاليًا، ويتم تطوير اختبارات أخرى.
كما أن الابتكارات والتقنيات المتزايدة والعدد المتزايد من اللاعبين في السوق وإطلاق المنتجات الجديدة تعمل أيضًا على دفع نمو سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا.
ديناميكيات سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
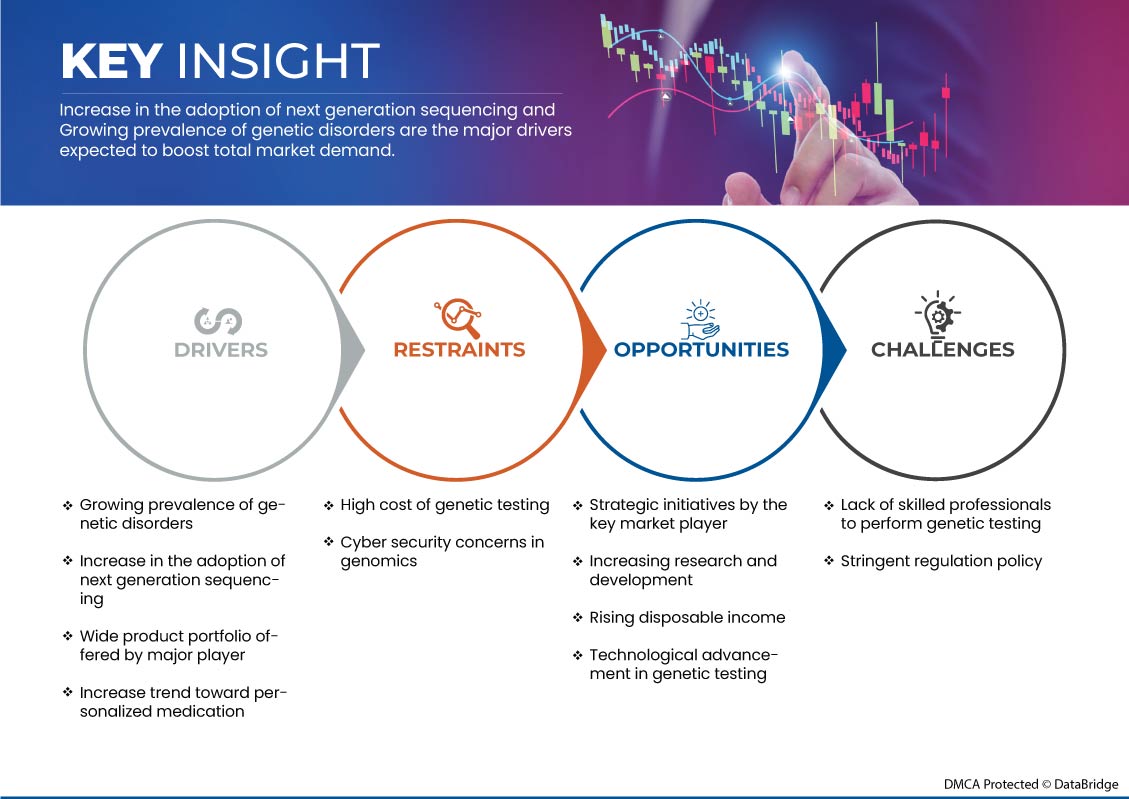
يتناول هذا القسم فهم محركات السوق والمزايا والفرص والقيود والتحديات. وسيتم مناقشة كل هذا بالتفصيل أدناه:
السائقون/الفرص
- انتشار متزايد للاضطرابات الوراثية
قد تتسبب الاضطرابات الوراثية في حدوث مشكلات صحية خطيرة لا تتوافق مع الحياة. وفي الحالات الأكثر شدة، قد تتسبب هذه الحالات في إجهاض الجنين المصاب. ويؤدي الانتشار المتزايد للأمراض الوراثية والعيوب الخلقية إلى زيادة الطلب على سوق الاختبارات الجينية.
- وبحسب مقال منظمة الصحة العالمية بعنوان "الاضطرابات الوراثية والتشوهات الخلقية: استراتيجيات للحد من العبء في المنطقة، 2022"، فإن الأمراض الوراثية والخلقية تشكل نسبة كبيرة من الوفيات قبل الولادة وحديثي الولادة في العديد من بلدان المنطقة. وتعتبر العيوب الخلقية الآن السبب الرئيسي لوفيات الرضع في الإمارات العربية المتحدة والسبب الثاني في البحرين والكويت وعمان وقطر. وتشير التقارير الواردة من المملكة العربية السعودية إلى أن حوالي 25-35٪ من الوفيات قبل الولادة في المستشفيين كانت بسبب عيوب خلقية.
ومن ثم، فإن هذا يؤدي إلى زيادة الطلب على سوق الاختبارات الجينية.
- زيادة في اعتماد تسلسل الجيل التالي
مع استمرار علم الأدوية الذي يركز على الجينوم في لعب دور أكبر في علاج العديد من الأمراض المزمنة، وخاصة السرطان، يتطور التسلسل الجيني للجيل التالي (NGS) كأداة قوية لتوفير نظرة أعمق وأكثر دقة في الأسس الجزيئية للأورام الفردية والمستقبلات المحددة.
تقدم تقنية الجيل التالي من الجينوم مزايا من حيث الدقة والحساسية والسرعة مقارنة بالطرق التقليدية التي لديها القدرة على إحداث تأثير كبير في مجال علم الأورام. ولأن تقنية الجيل التالي من الجينوم قادرة على تقييم جينات متعددة في اختبار واحد، فإن الحاجة إلى طلب اختبارات متعددة لتحديد الطفرة المسببة يتم التخلص منها.
ومن المتوقع أن يكون هذا بمثابة محرك لنمو سوق الاختبارات الجينية.
- ارتفاع الدخل المتاح
إن الإنفاق الذي تنفقه دولة ما على الرعاية الصحية ومعدل نموها بمرور الوقت يتأثر بمجموعة واسعة من العوامل الاقتصادية والاجتماعية، بما في ذلك ترتيبات التمويل وهيكل تنظيم النظام الصحي. وعلى وجه الخصوص، هناك ارتباط قوي بين مستوى الدخل الكامل لبلد ما ومقدار ما ينفقه سكان ذلك البلد على الرعاية الصحية.
كما أن المبادرات الاستراتيجية التي اتخذها اللاعبون الرئيسيون في السوق ستوفر السلامة البنيوية والفرص المستقبلية لسوق الاختبارات الجينية في الفترة المتوقعة 2023-2030.
القيود/التحديات
- ارتفاع تكلفة الاختبارات الجينية
قد تكون الاختبارات الجينية مكلفة وقد لا تغطيها بعض خطط التأمين الصحي. وتختلف تكاليف الاختبارات الجينية العديدة وفقًا للمرض المستهدف الذي يتم اختباره من أجله.
- وفقًا لموقع Breastcancer.org، يمكن أن تختلف تكلفة الاختبار الجيني للسرطان بشكل كبير ويمكن أن تتراوح بين 300 دولار أمريكي و5000 دولار أمريكي. يمكن أن تعتمد تكلفة الاختبار الجيني على نوع الاختبار بالإضافة إلى مدى تعقيده
- قد تتكلف الاختبارات الجينية ما بين 100 دولار أمريكي إلى أكثر من 2000 دولار أمريكي، وذلك حسب طبيعة الاختبار وتعقيده. وإذا كان الأمر يتطلب أكثر من اختبار واحد أو كان لابد من اختبار العديد من أفراد الأسرة للحصول على نتيجة مهمة، فإن التكلفة ترتفع. وتختلف تكلفة فحص حديثي الولادة حسب الولاية
ومن ثم، فإن التكلفة المرتفعة للاختبارات الجينية قد تحد من نمو السوق.
- سياسة تنظيمية صارمة
تلعب الشؤون التنظيمية دورًا حاسمًا في صناعة الأجهزة الطبية لأنها تهتم بدورة حياة المنتج الصحي. فهي تخدم المسارات التكتيكية والإستراتيجية والتشغيلية وتساعد في العمل ضمن اللوائح لتسريع تسليم وتطوير أجهزة ومنتجات اختبار اللعاب للسرطان الآمنة والفعالة للسكان في جميع أنحاء العالم. ويتمثل دور الشؤون التنظيمية في وضع وتنفيذ إستراتيجية تنظيمية.
تقدم العديد من منظمات الرعاية الصحية والهيئات الحكومية سياسات تنظيمية لإطلاق واعتماد أجهزة ومنتجات اختبار اللعاب الخاصة بالسرطان. ويلعب اعتماد المنتج من قبل الهيئات التنظيمية الإقليمية دورًا أساسيًا لفريق تطوير الأدوية. ويضمن الاعتماد أنشطة تطوير الأدوية التي تقوم بها الشركة ويميزها عن المنافسين.
تأثير ما بعد كوفيد-19 على سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
تأثر سوق الاختبارات الجينية بشدة بسبب جائحة كوفيد-19. فقد اقتصرت حالات الدخول إلى المستشفيات على العلاج غير الضروري، وأغلقت العيادات مؤقتًا أثناء الجائحة. كما أثر تطبيق التباعد الاجتماعي وحظر التجمعات والوصول المحدود إلى العيادات بشكل كبير على السوق. كما أثر تباطؤ تدفقات المرضى والإحالات على نمو السوق. ومع ذلك، سيستمر السوق في النمو في فترة ما بعد الجائحة بسبب تخفيف القيود المفروضة سابقًا.
يتخذ المصنعون قرارات استراتيجية مختلفة للتعافي بعد جائحة كوفيد-19. ويجري اللاعبون العديد من أنشطة البحث والتطوير وإطلاق المنتجات، ويقيمون شراكات استراتيجية لتحسين التكنولوجيا ونتائج الاختبارات التي تشارك فيها سوق الاختبارات الجينية.
التطورات الأخيرة
- في 10 نوفمبر 2022، أعلنت شركة Myriad Genetics, Inc.، الرائدة في مجال الاختبارات الجينية والطب الدقيق، عن إطلاق UroSuite، وهي مجموعة شاملة من اختبارات تقييم المخاطر الجينية التي تغطي جميع مراحل رعاية سرطان البروستاتا. تتضمن UroSuite اختبار سرطان البروستاتا Prolaris من Myriad، واختبار السرطان الوراثي MyRisk، واختبار BRACAnalysis CDx، واختبار Precise Tumor Molecular Profile. توفر مجموعة الاختبارات نظرة عامة جينية متكاملة وتسهل العلاج واختيار التجارب السريرية للمرضى.
- في سبتمبر 2022، أعلنت شركة Illumina, Inc.، وهي شركة رائدة عالميًا في تقنيات تسلسل الحمض النووي والصفائف، عن إطلاق سلسلة NovaSeq X (NovaSeq X وNovaSeq X Plus). هذه هي أجهزة تسلسل جديدة على نطاق الإنتاج تدفع حدود الطب الجينومي من خلال تمكين التسلسل بشكل أسرع وأكثر كفاءة واستدامة. بفضل التكنولوجيا الجديدة الثورية، يمكن لجهاز NovaSeq X Plus توليد أكثر من 20000 جينوم كامل سنويًا - وهو ما يزيد بمقدار 2.5 مرة عن أداء أجهزة التسلسل السابقة - مما يسرع بشكل كبير من اكتشاف الجينوم والرؤى السريرية لفهم المرض وفي النهاية تحويل حياة المرضى.
نطاق سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
يتم تقسيم سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا إلى نوع وتقنية وأمراض ومستخدم نهائي. سيساعدك النمو بين هذه القطاعات على تحليل وقياس قطاعات النمو في الصناعات وتزويد المستخدمين بنظرة عامة قيمة على السوق ورؤى السوق لاتخاذ قرارات استراتيجية لتحديد تطبيقات السوق الأساسية.
يكتب
- الاختبارات التشخيصية
- اختبارات ما قبل الولادة
- فحص حديثي الولادة
- الاختبارات التنبؤية والاختبارات ما قبل الأعراض
- اختبار الناقل
- أنواع أخرى
على أساس النوع، يتم تقسيم سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا إلى اختبارات تشخيصية، واختبارات ما قبل الولادة، وفحص حديثي الولادة، والاختبارات التنبؤية والأعراضية، واختبار الناقل وأنواع أخرى.
تكنولوجيا
- تفاعل البوليميراز المتسلسل
- تسلسل الحمض النووي (اختبار يعتمد على NGS)
- تسلسل الجينوم الكامل
- المصفوفات الدقيقة
- التهجين الموضعي الفلوري (FISH)
- آحرون
على أساس التكنولوجيا، يتم تقسيم سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا إلى تسلسل الحمض النووي، وتفاعل البوليميراز المتسلسل، والمصفوفات الدقيقة، وتسلسل الجينوم الكامل، والتهجين الموضعي الفلوري (FISH)، وغيرها.
الأمراض
- سرطان
- فقر الدم المنجلي
- الثلاسيميا
- اضطراب وراثي نادر
- متلازمة إكس الهش
- ضمور العضلات دوشين
- مرض هنتنغتون
- تليّف كيسي
- الاختبارات الجينية الإنجابية
- الاختبارات الجينية للصحة والعافية
- آحرون
على أساس الأمراض، يتم تقسيم سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا إلى اضطراب وراثي نادر ، والسرطان، والتليف الكيسي، وفقر الدم المنجلي، وخلل العضلات دوشين، والثلاسيميا، ومرض هنتنغتون، ومتلازمة الصبغي X الهش، والاختبارات الجينية التناسلية، والاختبارات الجينية للصحة والعافية، وغيرها.
المستخدم النهائي
- المستشفيات
- العيادات
- مراكز التشخيص
- العيادات الخاصة
- مقدمي خدمات المختبر
- المختبرات الخاصة
على أساس المستخدمين النهائيين، يتم تقسيم سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا إلى المستشفيات والعيادات ومراكز التشخيص والعيادات الخاصة ومقدمي الخدمات المعملية والمختبرات الخاصة.
تحليل/رؤى إقليمية لسوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
يتم تحليل سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا، ويتم توفير معلومات حجم السوق حسب النوع والتكنولوجيا والأمراض والمستخدم النهائي.
الدول التي يغطيها تقرير السوق هذا هي جنوب أفريقيا والمملكة العربية السعودية والإمارات العربية المتحدة ومصر وإسرائيل وبقية دول الشرق الأوسط وأفريقيا.
ومن المتوقع أن تهيمن جنوب أفريقيا في عام 2023 بسبب الاستثمار المتزايد في البحث والتطوير ومن المتوقع أن يعزز نمو السوق.
كما يقدم قسم الدولة في التقرير العوامل الفردية المؤثرة على السوق والتغيرات في التنظيم في السوق محليًا والتي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. تعد نقاط البيانات مثل المبيعات الجديدة ومبيعات الاستبدال والتركيبة السكانية للدولة والقوانين التنظيمية ورسوم الاستيراد والتصدير من بين المؤشرات الرئيسية المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. كما يتم النظر في وجود وتوافر العلامات التجارية في الشرق الأوسط وأفريقيا والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية وتأثير قنوات المبيعات أثناء تقديم تحليل تنبؤي لبيانات الدولة.
تحليل المشهد التنافسي وحصة سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا
يقدم المشهد التنافسي لسوق الاختبارات الجينية في الشرق الأوسط وأفريقيا تفاصيل عن كل منافس. تتضمن التفاصيل نظرة عامة على الشركة، والبيانات المالية للشركة، والإيرادات المتولدة، وإمكانات السوق، والاستثمار في البحث والتطوير، ومبادرات السوق الجديدة، ومواقع الإنتاج والمرافق، ونقاط القوة والضعف في الشركة، وإطلاق المنتج، وخطوط أنابيب تجارب المنتجات، وموافقات المنتج، وبراءات الاختراع، وعرض المنتج وتنفسه، وهيمنة التطبيق، ومنحنى شريان الحياة التكنولوجي. تتعلق نقاط البيانات المذكورة أعلاه فقط بتركيز الشركة على سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا.
بعض اللاعبين الرئيسيين العاملين في سوق الاختبارات الجينية في الشرق الأوسط وأفريقيا هم Abbott، و 23ANDME، Inc.، و Danaher، و Myriad Genetics، Inc.، و OCP Medical Centre، LLC، و Healthchecks360، و Bayer AG، و BioReference Health، LLC (شركة تابعة لشركة OPKO Health، Inc.)، و FREIBURG MEDICAL LABORATORY MIDDLE EAST (LLC)، و Thermo Fisher Scientific Inc.، و Bio-Rad Laboratories، Inc.، و Biocartis، و Eurofins Scientific، و PerkinElmer Inc.، و ELITechGroup، و F. Hoffmann-La Roche Ltd.، و Illumina، Inc.، و QIAGEN، و BIO-HELIX، و PacBio وغيرها.
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. بالإضافة إلى ذلك، يعد تحليل حصة السوق وتحليل الاتجاهات الرئيسية من عوامل النجاح الرئيسية في تقرير السوق. منهجية البحث الرئيسية التي يستخدمها فريق البحث في DBMR هي التثليث البيانات والتي تنطوي على استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (خبير الصناعة). وبصرف النظر عن هذا، تتضمن نماذج البيانات شبكة وضع البائعين، وتحليل الخط الزمني للسوق، ونظرة عامة على السوق والدليل، وشبكة وضع الشركة، وتحليل حصة الشركة في السوق، ومعايير القياس، والشرق الأوسط وأفريقيا مقابل المنطقة، وتحليل حصة البائعين. يرجى طلب مكالمة محلل في حالة وجود استفسار آخر.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 YEARS CONSIDERED FOR THE STUDY
2.3 CURRENCY AND PRICING
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL’S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 STRATEGIC INITIATIVES:
5 INDUSTRY INSIGHTS
5.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.2 CANCER GENETICS RISK ASSESSMENT AND COUNSELING
5.3 GENETIC TESTS PRICING AND KEY PRICING STRATEGIES
5.3.1 LAUNCH OF NEW TECHNOLOGY GENETIC TESTING KITS
5.3.2 PARTNERSHIPS WITH MARKET PLAYERS
5.3.3 PRENATAL PRICING STRATEGY
5.4 KEY PATIENT ENROLLMENT STRATEGIES
5.4.1 AWARENESS OF THE PUBLIC TOWARDS GENETIC TESTING TECHNOLOGY
5.4.2 GENETIC COUNSELLORS' SCOPE OF PRACTICE
5.4.3 EDUCATION AND COMMUNICATION
6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: REGULATIONS
7 EPIDEMIOLOGY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GENETIC DISORDERS
8.1.2 INCREASE IN THE ADOPTION OF NEXT GENERATION SEQUENCING
8.1.3 WIDE PRODUCT PORTFOLIO OFFERED BY MAJOR PLAYER
8.1.4 INCREASE TREND TOWARD PERSONALIZED MEDICATION
8.2 RESTRAINT
8.2.1 HIGH COST OF GENETIC TESTING
8.2.2 CYBER SECURITY CONCERN IN GENOMICS
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 TECHNOLOGICAL ADVANCEMENTS IN GENETIC TESTING
8.3.3 INCREASING RESEARCH AND DEVELOPMENT
8.3.4 RISING DISPOSABLE INCOME
8.4 CHALLENGES
8.4.1 LACK OF SKILLED PROFESSIONALS TO PERFORM GENETIC TESTING
8.4.2 STRINGENT REGULATION POLICY
9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE
9.1 OVERVIEW
9.2 DIAGNOSTIC TESTING
9.3 PRENATAL TESTING
9.3.1 NON-INVASIVE SCREENING
9.3.1.1 BY SCREENING METHOD
9.3.1.1.1 WHOLE GENOME SEQUENCING
9.3.1.1.2 COUNTING OF CFDNA FRAGMENTS
9.3.1.1.3 OTHERS
9.3.1.2 BY CONDITION
9.3.1.2.1 TRISOMY 21
9.3.1.2.2 KLINEFELTER SYNDROME
9.3.1.2.3 JACOBS SYNDROME
9.3.1.2.4 CYSTIC FIBROSIS
9.3.1.2.5 TURNER SYNDROME
9.3.1.2.6 TRISOMY 18
9.3.1.2.7 HEMOPHILIA
9.3.1.2.8 TRISOMY 13
9.3.1.2.9 MICRODELETION SYNDROME
9.3.1.2.10 FETAL GENDER
9.3.1.2.11 OTHERS
9.3.1.3 BY SCREENING TYPE
9.3.1.3.1 CARRIER SEQUENCING
9.3.1.3.2 SEQUENTIAL SEQUENCING
9.3.2 MATERNAL SERUM QUAD SCREENING
9.4 NEW BORN SCREENING
9.4.1 SICKLE CELL DISEASE
9.4.2 CONGENITAL HYPOTHYROIDISM
9.4.3 PHENYLKETONURINA (PKU)
9.4.4 GALACTOSEMIA
9.4.5 MAPLE SYRUP URINE DISEASE
9.4.6 OTHERS
9.5 PREDICTIVE AND PRESYMPTOMATIC TESTING
9.6 CARRIER TESTING
9.6.1 BY TEST TYPE
9.6.1.1 MOLECULAR SCREENING TEST
9.6.1.2 BIOCHEMICAL SCREENING TEST
9.6.2 BY TYPE
9.6.2.1 EXPANDED CARRIER SCREENING
9.6.2.1.1 PREDESIGNED PANEL TESTING
9.6.2.1.2 CUSTOM-MADE PANEL TESTING
9.6.2.2 TARGETED DISEASE CARRIER SCREENING
9.6.3 BY MEDICAL CONDITION
9.6.3.1 HEMATOLOGICAL CONDITIONS
9.6.3.2 PULMONARY CONDITIONS
9.6.3.3 NEUROLOGICAL CONDITIONS
9.6.3.4 OTHER CONDITIONS
9.7 OTHER TYPES
10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY
10.1 OVERVIEW
10.2 POLYMERASE CHAIN REACTION
10.2.1 REAL-TIME PCR (QPCR)
10.2.2 DIGITAL PCR (DPCR)
10.2.3 REVERSE TRANSCRIPTION PCR (RT-PCR)
10.2.4 HOT-START PCR
10.2.5 MULTIPLEX PCR
10.2.6 OTHER PCR
10.3 DNA SEQUENCING (NGS-BASED TESTING)
10.3.1 NEXT GENERATION SEQUENCING (NGS)
10.3.2 SANGER SEQUENCING (SINGLE GENE)
10.3.3 OTHER
10.4 WHOLE GENOME SEQUENCING
10.5 MICROARRAYS
10.5.1 DNA MICROARRAYS
10.5.2 PROTEIN MICROARRAYS
10.5.3 OTHER MICROARRAYS
10.6 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
10.7 OTHERS
11 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY DISEASES
11.1 OVERVIEW
11.2 CANCER
11.2.1 BREAST
11.2.2 COLON
11.2.3 LUNG
11.2.4 PROSTATE
11.2.5 OTHERS
11.3 REPRODUCTIVE GENETIC TESTING
11.4 HEALTH AND WELLNESS GENETIC TESTING
11.5 SICKLE CELL ANEMIA
11.6 THALASSEMIA
11.7 RARE GENETIC DISORDER
11.7.1 TRISOMY 21
11.7.2 MONOSOMY X
11.7.3 TRISOMY 13
11.7.4 MICRODELETION SYNDROME
11.7.5 TRISOMY 18
11.7.6 OTHERS
11.8 FRAGILE X SYNDROME
11.9 DUCHENNE MUSCULAR DYSTROPHY
11.1 HUNTINGTON'S DISEASE
11.11 CYSTIC FIBROSIS
11.12 OTHERS
12 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 CLINICS
12.4 DIAGNOSTIC CENTERS
12.5 PRIVATE CLINICS
12.6 LABORATORY SERVICE PROVIDERS
12.7 PRIVATE LABORATORIES
13 SUMMARY WRITE-UP (MIDDLE EAST AND AFRICA)
13.1 OVERVIEW
13.2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY
13.2.1 SOUTH AFRICA
13.2.2 SAUDI ARABIA
13.2.3 U.A.E.
13.2.4 ISRAEL
13.2.5 EGYPT
13.2.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 BAYER AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENTS
16.2 ABBOTT
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 ILLUMINA, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 THERMO FISHER SCIENTIFIC INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 F.HOFFAMANN-LA ROCHE LTD.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 BIOREFERNCE HEALTH INC. (SUBSIDIARY OPKO HEALTH, INC.)
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 ELITECHGROUP
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 23ANDME, INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 BIOCARTIS
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 BIO-HELIX
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 BIO-RAD LABORATORIES, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 DANAHER
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 EUROFINS SCIENTIFIC
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENTS
16.14 FREIBURG MEDICAL LABORATORY MIDDLE EAST (L.L.C)
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 HEALTHCHECKS360
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
16.16 MYRIAD GENETICS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENTS
16.17 OCP MEDICAL CENTER L.L.C
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 PACBIO
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 PERKINELMER ONC.
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENTS
16.2 QIAGEN
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 4 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 6 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 7 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 9 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 10 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 13 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 14 MIDDLE EAST AND AFRICA NEW BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 17 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 18 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 20 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 21 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 23 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 24 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET , BY DISEASES, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 33 IDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY 2021-2030 (USD MILLION)
TABLE 34 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 36 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 37 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 38 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 39 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 40 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 41 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 42 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 43 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 44 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 45 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 46 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 47 SOUTH AFRICA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 50 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 51 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 53 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 54 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 55 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 56 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 57 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 58 SOUTH AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 59 SOUTH AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 60 SOUTH AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 61 SOUTH AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 62 SOUTH AFRICA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 63 SOUTH AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 64 SOUTH AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 65 SOUTH AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 66 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 68 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 69 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 71 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 72 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 73 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 74 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 75 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 76 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 77 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 78 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 79 SAUDI ARABIA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 81 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 82 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 83 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 85 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 86 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 87 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 88 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 89 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 90 SAUDI ARABIA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 SAUDI ARABIA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 92 SAUDI ARABIA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 93 SAUDI ARABIA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 94 SAUDI ARABIA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 95 SAUDI ARABIA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 96 SAUDI ARABIA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 97 SAUDI ARABIA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 98 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 100 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 101 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 103 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 104 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 105 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 106 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 107 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 108 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 109 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 110 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 111 U.A.E. NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 112 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 114 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 115 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 117 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 118 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 120 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 121 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 122 U.A.E. GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.A.E. POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 124 U.A.E. DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 125 U.A.E. MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 126 U.A.E. GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 127 U.A.E. RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 128 U.A.E. CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 129 U.A.E. GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 132 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 133 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 135 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 136 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 137 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 138 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 139 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 140 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 141 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 142 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 143 ISRAEL NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 145 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 146 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 147 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 148 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 149 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 150 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 152 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 153 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 154 ISRAEL GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 ISRAEL POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 156 ISRAEL DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 157 ISRAEL MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 158 ISRAEL GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 159 ISRAEL RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 160 ISRAEL CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 161 ISRAEL GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 162 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 163 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 164 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 165 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 167 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 168 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 169 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 170 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 171 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 172 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 173 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 174 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 175 EGYPT NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 177 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 178 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 180 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 181 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 183 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 184 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 185 EGYPT GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 186 EGYPT POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 EGYPT DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 188 EGYPT MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 189 EGYPT GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 190 EGYPT RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 191 EGYPT CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 192 EGYPT GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 REST OF MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET:DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET :COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : MARKET APPLICATION COVERAGE GRID
FIGURE 9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 11 THE INCREASING PREVALENCE OF GENETIC DISEASES AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET GROWTH IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 DIGANOSTIC TESTING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET IN 2023 & 2030
FIGURE 13 EPIDEMIOLOGY
FIGURE 14 INCIDENCE OF ALL GENDER-SOUTH AFRICA AND SAUDI ARABIA
FIGURE 15 INCIDENCE OF ALL GENDER-UAE AND EGYPT
FIGURE 16 INCIDENCE OF ALL GENDER-ISRAEL
FIGURE 17 MORTALITY RATE- SOUTH AFRICA AND SAUDI ARABIA
FIGURE 18 MORTALITY RATE-UAE AND EGYPT
FIGURE 19 MORTALITY RATE-ISRAEL
FIGURE 20 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
FIGURE 21 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2022
FIGURE 22 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 23 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 24 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2022
FIGURE 26 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 27 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 28 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2022
FIGURE 30 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 31 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 33 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2022
FIGURE 34 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 35 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, CAGR (2023-2030)
FIGURE 36 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SNAPSHOT (2022)
FIGURE 38 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022)
FIGURE 39 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 40 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE (2023-2030)
FIGURE 42 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: COMPANY SHARE 2022 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.













