Global Pcr Based Infectious Diseases Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
5.05 Billion
USD
8.42 Billion
2024
2032
USD
5.05 Billion
USD
8.42 Billion
2024
2032
| 2025 –2032 | |
| USD 5.05 Billion | |
| USD 8.42 Billion | |
|
|
|
|
تجزئة سوق الأمراض المعدية العالمية القائمة على تفاعل البوليميراز المتسلسل (PCR)، حسب نوع الاختبار (اختبار السل، اختبار التهاب الكبد الوبائي، اختبار فيروس نقص المناعة البشرية (HIV)، اختبار الإنفلونزا، اختبار فيروس الورم الحليمي البشري (HPV)، اختبار السيلان، اختبار السالمونيلا، اختبار فيروس الروتا، اختبار البورديتيلا، اختبار الملوية البوابية، اختبار نوروفيروس، اختبار السارس (كوفيد-19)، اختبار المعوية، اختبار الكلاميديا التراخوماتية التناسلية، اختبار المطثية العسيرة (Clostridium difficile)، اختبار المعوية المقاومة للكاربابينيم (CRE)، اختبار الميكوبلازما التناسلية (MG)، اختبار المكورات العنقودية الذهبية المقاومة للميثيسيلين (MRSA)، واختبارات أخرى. الاختبار)، العدوى (العدوى الفيروسية، عدوى الجهاز التنفسي، العدوى المنقولة جنسيًا، العدوى المكتسبة من المستشفيات، وغيرها من العدوى)، مسببات الأمراض (الفيروسية، البكتيرية، الفطرية، الأوليات، وغيرها)، تقنية تفاعل البوليميراز المتسلسل (تفاعل البوليميراز المتسلسل العكسي، تفاعل البوليميراز المتسلسل المتعدد، وغيرها)، نوع المريض (كبار السن، الأطفال، والبالغين)، الاختبار (الاختبارات المعملية واختبارات نقطة الرعاية)، المستخدم النهائي (مراكز التشخيص، المستشفيات، المعاهد الأكاديمية والبحثية، مراكز الصحة المجتمعية، العيادات، الرعاية الصحية المنزلية، وغيرها) - اتجاهات الصناعة والتوقعات حتى عام 2032
حجم سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
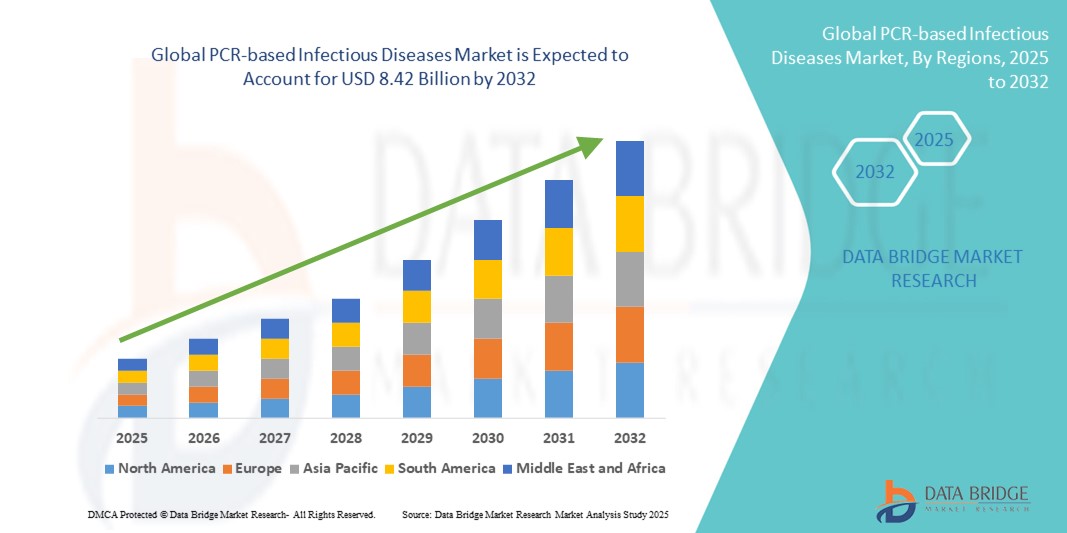
- تم تقييم حجم سوق الأمراض المعدية العالمية القائمة على تفاعل البوليميراز المتسلسل (PCR) بنحو 5.05 مليار دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 8.42 مليار دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 6.60٪ خلال الفترة المتوقعة.
- يُعزى نمو السوق بشكل كبير إلى تزايد اعتماد منصات التشخيص الجزيئي والتقدم التكنولوجي فيها، لا سيما الاستخدام الواسع النطاق لتقنية تفاعل البوليميراز المتسلسل (PCR) في الوقت الفعلي للكشف عن الأمراض المعدية بحساسية ودقة عاليتين. وقد أدى تزايد انتشار أمراض مثل الإنفلونزا والسل وفيروس نقص المناعة البشرية والتهاب الكبد ومسببات الأمراض الناشئة إلى زيادة الطلب السريري على أدوات تشخيص سريعة ودقيقة وقابلة للتطوير.
- علاوة على ذلك، يُسهم الوعي العالمي المتزايد بالكشف المبكر عن الأمراض، إلى جانب توسع مرافق التشخيص اللامركزية، مثل مختبرات الرعاية الصحية ووحدات الاختبار المتنقلة، في تسريع اعتماد حلول الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR). هذه العوامل المتقاربة - مدعومة بمبادرات الصحة العامة، وتحسين البنية التحتية للرعاية الصحية، وتزايد الاستثمار في مراقبة الأمراض المعدية - تُعزز نمو هذه الصناعة بشكل كبير في الأسواق المتقدمة والناشئة على حد سواء.
تحليل سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR)
- أصبحت أدوات التشخيص القائمة على تفاعل البوليميراز المتسلسل، والتي تمكن من الكشف السريع والدقيق عن مسببات الأمراض المعدية من خلال تضخيم المادة الوراثية، ذات أهمية متزايدة في جميع البيئات السريرية والبحثية بسبب حساسيتها العالية وسرعتها وقدرتها على اكتشاف مجموعة واسعة من العوامل المعدية، بما في ذلك الفيروسات والبكتيريا والطفيليات.
- إن العبء العالمي المتزايد للأمراض المعدية، إلى جانب الوعي المتزايد في أعقاب الأوبئة الأخيرة، يدفع الطلب بشكل كبير على التقنيات القائمة على تفاعل البوليميراز المتسلسل، وخاصة في تشخيص أمراض مثل كوفيد-19 والسل والتهاب الكبد والأمراض المنقولة جنسياً.
- سيطرت أمريكا الشمالية على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) بحصة إيرادات بلغت 38.9% في عام 2024، مدفوعةً ببنية تحتية قوية للرعاية الصحية، وقدرات تشخيصية فعّالة، واعتماد مبكر لأدوات التشخيص الجزيئي المتقدمة. وشهدت الولايات المتحدة، على وجه الخصوص، نموًا ملحوظًا بفضل المبادرات الحكومية، وارتفاع أعداد الاختبارات، والابتكار التكنولوجي المستمر.
- من المتوقع أن تكون منطقة آسيا والمحيط الهادئ أسرع منطقة نموًا في سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل بمعدل نمو سنوي مركب يبلغ 11.3٪ من عام 2025 إلى عام 2032، وذلك بسبب توسيع نطاق الوصول إلى الرعاية الصحية، وارتفاع الإنفاق على الرعاية الصحية، والوعي المتزايد بالتشخيص المبكر للأمراض المعدية في البلدان النامية بسرعة مثل الصين والهند ودول جنوب شرق آسيا.
- سيطرت شريحة الاختبارات المعملية على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) بحصة بلغت 68.9% في عام 2024، بدعم من البنية التحتية المركزية للمختبرات وقدرة المعالجة بالجملة
نطاق التقرير وتجزئة سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
|
صفات |
رؤى السوق الرئيسية للأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
أمريكا الشمالية
أوروبا
آسيا والمحيط الهادئ
الشرق الأوسط وأفريقيا
أمريكا الجنوبية
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تشمل أيضًا تحليلًا متعمقًا من الخبراء وتحليل التسعير وتحليل حصة العلامة التجارية واستطلاع رأي المستهلكين وتحليل التركيبة السكانية وتحليل سلسلة التوريد وتحليل سلسلة القيمة ونظرة عامة على المواد الخام / المواد الاستهلاكية ومعايير اختيار البائعين وتحليل PESTLE وتحليل Porter والإطار التنظيمي. |
اتجاهات سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR)
زيادة الكفاءة والتخصيص في حلول التشخيص
- إن الاتجاه البارز الذي يشكل سوق الأمراض المعدية العالمية القائمة على تفاعل البوليميراز المتسلسل هو التقدم ودمج الأتمتة الذكية والتقنيات المتصلة التي تعمل على تبسيط سير عمل التشخيص وتعزيز الدقة
- على سبيل المثال، تتكامل منصات تفاعل البوليميراز المتسلسل (PCR) الآلية الآن مع برامج متقدمة قادرة على التنبؤ بأنماط العدوى، وتوفير تحليلات بيانات آنية، وتبسيط الاختبارات واسعة النطاق. وتُعد هذه التحسينات مؤثرة بشكل خاص في البيئات عالية الإنتاجية، مثل المستشفيات والمختبرات ومراكز اختبارات الصحة العامة.
- توفر العديد من أنظمة تفاعل البوليميراز المتسلسل الحديثة الآن اتصالاً قائمًا على السحابة يسمح بالمراقبة عن بُعد وإنشاء التقارير والتكامل السلس مع السجلات الصحية الإلكترونية (EHRs)، مما يحسن سرعة وتنسيق إدارة الأمراض المعدية
- بالإضافة إلى ذلك، فإن التبني المتزايد لأنظمة تفاعل البوليميراز المتسلسل في نقطة الرعاية (POC) يحول التشخيص في البيئات الريفية أو ذات الموارد المحدودة من خلال تقديم نتائج سريعة بجودة المختبر دون الحاجة إلى البنية التحتية المركزية للمختبر
- تعمل شركات مثل Roche وCepheid وBio-Rad باستمرار على ترقية منصات الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) من خلال واجهات سهلة الاستخدام وقدرات إرسال متعددة محسنة - مما يتيح الكشف المتزامن عن مسببات الأمراض المتعددة من عينة واحدة
- يؤدي التقارب بين الأتمتة والاتصال المعتمد على البرمجيات إلى إعادة تعريف توقعات المستخدمين لتشخيص الأمراض المعدية من خلال تقديم حلول مخصصة وسريعة وأكثر سهولة في الوصول عبر مختلف إعدادات الرعاية الصحية، وبالتالي تسريع التبني العالمي للتقنيات القائمة على تفاعل البوليميراز المتسلسل.
ديناميكيات سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR)
سائق
الحاجة المتزايدة بسبب ارتفاع عبء العدوى والطلب على التشخيص
- إن العبء العالمي المتزايد للأمراض المعدية، إلى جانب الحاجة إلى أدوات تشخيصية سريعة ودقيقة، هو المحرك الرئيسي الذي يغذي الطلب على حلول الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
- على سبيل المثال، أفادت منظمة الصحة العالمية (WHO) بارتفاع حاد في حالات الإصابة بمرض السل عالميًا، حيث يُقدر أن يُصاب به 10.6 مليون شخص في عام 2022. أصبحت اختبارات التشخيص القائمة على تفاعل البوليميراز المتسلسل (PCR)، مثل جين إكسبرت، بالغة الأهمية للكشف السريع عن السل والسلالات المقاومة للأدوية، مما يُحسّن نتائج العلاج بشكل كبير ويُقلل من انتقال العدوى. يُبرز هذا الاعتماد المتزايد على تفاعل البوليميراز المتسلسل للكشف عن الأمراض المعدية دوره المتنامي في أنظمة الرعاية الصحية العالمية.
- مع مواجهة أنظمة الرعاية الصحية لضغوط متزايدة للكشف عن العدوى واحتوائها بسرعة - وخاصة في المستشفيات ومؤسسات الصحة العامة - توفر تقنية تفاعل البوليميراز المتسلسل تحديدًا دقيقًا لمسببات الأمراض، وحدود اكتشاف منخفضة، وأوقات استجابة سريعة، متفوقة بشكل كبير على الطرق التقليدية القائمة على الثقافة.
- علاوةً على ذلك، يُتيح تزايد شعبية أنظمة تفاعل البوليميراز المتسلسل (PCR) في نقاط الرعاية (POC) في العيادات المجتمعية، ورعاية الطوارئ، والأماكن النائية، وصولاً أوسع للتشخيص المبكر والعلاج. ويتماشى هذا التعميم في إجراء الاختبارات مع مبادرات الصحة العالمية التي تُركز على التأهب للأوبئة وتوفير الرعاية الصحية في المناطق الريفية.
- تُعدّ سهولة سير عمل تفاعل البوليميراز المتسلسل (PCR) من العينة إلى الإجابة، وأتمتة مجموعات مسببات الأمراض، وقدرات المراقبة الفورية، من الميزات الرئيسية التي تُعزز اعتمادها في المستشفيات ومختبرات التشخيص، وحتى في سيناريوهات الفحص المنزلي. كما أن التوجه المتزايد نحو التشخيص اللامركزي والمنصات سهلة الاستخدام يُعزز نمو السوق.
ضبط النفس/التحدي
المخاوف بشأن القدرة على تحمل التكاليف وخصوصية البيانات
- على الرغم من فوائدها، لا تزال التكاليف المرتفعة المرتبطة بأجهزة التشخيص والمواد الاستهلاكية القائمة على تفاعل البوليميراز المتسلسل (PCR) تشكل عائقًا كبيرًا، لا سيما في البلدان منخفضة ومتوسطة الدخل. ويمكن لقيود الميزانية في أنظمة الرعاية الصحية أن تحد من إمكانية الوصول إلى الاختبارات الجزيئية المتقدمة، مما يعيق تطبيقها على نطاق واسع.
- بالإضافة إلى ذلك، تُعدّ المخاوف المتزايدة بشأن خصوصية بيانات المرضى والأمن السيبراني في منصات التشخيص المتصلة تحدياتٍ ناشئة. يجب أن تستوفي أنظمة تفاعل البوليميراز المتسلسل السحابية، التي تنقل بيانات المرضى أو تخزنها عن بُعد، لوائح الامتثال الصارمة، وأي خرق قد يُضعف ثقة المستخدم.
- على سبيل المثال، أثارت التقارير الأخيرة عن ثغرات الأمن السيبراني في منصات التشخيص المتصلة مخاوف بشأن حماية البيانات، وخاصة عندما يتم دمج الأنظمة مع شبكات المعلومات الصحية الأوسع.
- لمعالجة هذه المشكلات، تُعطي الشركات الأولوية لتشفير البيانات، وبروتوكولات الوصول الآمن، وتحديثات البرامج الثابتة بانتظام. كما تُسهم الجهود التثقيفية حول معالجة البيانات والشفافية في بناء الثقة بين مقدمي الرعاية الصحية والمستخدمين النهائيين.
- أخيرًا، على الرغم من أن أسعار التقنيات القائمة على تفاعل البوليميراز المتسلسل تتراجع تدريجيًا بسبب الحجم والابتكار، فإن التكلفة المرتفعة المتوقعة مقارنة بالاختبارات السريعة القائمة على المستضدات قد تعيق استخدامها في برامج الفحص الشامل أو التطبيقات غير الحرجة.
- سيتطلب التغلب على هذه التحديات جهودًا تعاونية بين مطوري التكنولوجيا والهيئات التنظيمية ومنظمات الصحة العامة لتحسين كفاءة التكلفة وضمان الامتثال التنظيمي وتعزيز الوصول العادل إلى تشخيصات تفاعل البوليميراز المتسلسل المتطورة.
نطاق سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
يتم تقسيم السوق على أساس نوع الاختبار والعدوى والممرض وتكنولوجيا تفاعل البوليميراز المتسلسل ونوع المريض والاختبار والمستخدم النهائي.
- حسب نوع الاختبار
على أساس نوع الاختبار، يتم تقسيم سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل إلى اختبار تفاعل البوليميراز المتسلسل للسل، واختبار تفاعل البوليميراز المتسلسل لالتهاب الكبد، واختبار تفاعل البوليميراز المتسلسل لفيروس نقص المناعة البشرية (HIV)، واختبار تفاعل البوليميراز المتسلسل للإنفلونزا، واختبار تفاعل البوليميراز المتسلسل لفيروس الورم الحليمي البشري (HPV)، واختبار تفاعل البوليميراز المتسلسل للسيلان، واختبار تفاعل البوليميراز المتسلسل للسالمونيلا، واختبار تفاعل البوليميراز المتسلسل للفيروس العجلي، واختبار تفاعل البوليميراز المتسلسل للبورديتيلا، واختبار تفاعل البوليميراز المتسلسل للجرثومة الحلزونية البوابية، واختبار تفاعل البوليميراز المتسلسل للنوروفيروس، واختبار تفاعل البوليميراز المتسلسل للسارس (كوفيد-19)، واختبار تفاعل البوليميراز المتسلسل للمكورات المعوية، واختبار تفاعل البوليميراز المتسلسل للأعضاء التناسلية للكلاميديا التراخوماتية، واختبار تفاعل البوليميراز المتسلسل للمطثية العسيرة (كلوستريديوم ديفيسيل)، واختبار تفاعل البوليميراز المتسلسل للبكتيريا المعوية المقاومة للكاربابينيم (CRE)، واختبار تفاعل البوليميراز المتسلسل للميكوبلازما التناسلية (MG)، واختبار تفاعل البوليميراز المتسلسل للمكورات العنقودية الذهبية المقاومة للميثيسيلين (MRSA)، واختبار تفاعل البوليميراز المتسلسل الآخر. سيطرت شريحة اختبار تفاعل البوليميراز المتسلسل لمرض السارس (كوفيد-19) على السوق بحصة إيرادات بلغت 29.3% في عام 2024، مدفوعًا بالبنية التحتية المستدامة للاختبار بعد الوباء والطلب المستمر على تشخيصات الجهاز التنفسي.
من المتوقع أن يسجل قطاع اختبار تفاعل البوليميراز المتسلسل لفيروس الورم الحليمي البشري (HPV) أسرع معدل نمو سنوي مركب بنسبة 11.8% من عام 2025 إلى عام 2032، وذلك بسبب الوعي المتزايد بشأن فحص سرطان عنق الرحم ومبادرات الصحة العامة الأوسع.
- عن طريق العدوى
بناءً على نوع العدوى، يُقسّم سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) إلى عدوى فيروسية، وعدوى الجهاز التنفسي، والعدوى المنقولة جنسيًا، والعدوى المكتسبة من المستشفيات، وغيرها من أنواع العدوى. وقد استحوذ قطاع العدوى الفيروسية على الحصة الأكبر بنسبة 42.1% في عام 2024، مدعومًا بارتفاع حجم اختبارات كوفيد-19، والتهاب الكبد، وفيروس نقص المناعة البشرية (HIV).
من المتوقع أن يشهد قطاع العدوى المكتسبة من المستشفيات أسرع معدل نمو سنوي مركب بنسبة 10.2% من عام 2025 إلى عام 2032، مدفوعًا بالتركيز التنظيمي المتزايد على مكافحة العدوى في مؤسسات الرعاية الصحية.
- حسب الممرض
بناءً على مُسبِّب المرض، يُقسَّم سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) إلى فيروسات، وبكتريا، وفطريات، وأوليات، وغيرها. وقد تصدَّر قطاع مُسبِّبات الأمراض الفيروسية السوق بحصة بلغت 45.7% في عام 2024، إذ لا يزال تفاعل البوليميراز المتسلسل (PCR) هو المعيار الأمثل للكشف عن الحمض النووي الريبوزي الفيروسي (RNA).
ومن المتوقع أن ينمو قطاع البكتيريا بمعدل نمو سنوي مركب نسبته 9.7% من عام 2025 إلى عام 2032، مدفوعًا بالحاجة إلى التشخيص السريع في ظل تزايد مقاومة المضادات الحيوية.
- بواسطة تقنية تفاعل البوليميراز المتسلسل
بناءً على التكنولوجيا، يُقسّم سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) إلى تفاعل البوليميراز المتسلسل العكسي (RT-PCR)، وتفاعل البوليميراز المتسلسل المتعدد (Multiplex PCR)، وغيرها. وسيستحوذ هذا القطاع على الحصة الأكبر (53.5%) في عام 2024، بفضل حساسيته العالية وانتشار استخدامه.
من المتوقع أن يتوسع قطاع تفاعل البوليميراز المتسلسل المتعدد بأسرع معدل نمو سنوي مركب بنسبة 12.6% من عام 2025 إلى عام 2032، وذلك بفضل كفاءته في الكشف المتزامن عن مسببات الأمراض المتعددة.
- حسب نوع المريض
بناءً على نوع المريض، يُقسّم سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) إلى فئات: كبار السن، والأطفال، والبالغين. وسيستحوذ البالغون على الحصة الأكبر بنسبة 48.3% في عام 2024، حيث تستهدف معظم فحوصات الأمراض المنقولة جنسيًا، والتهاب الكبد، والتهابات الجهاز التنفسي هذه الفئة السكانية.
ومن المتوقع أن يسجل قطاع طب الأطفال معدل نمو سنوي مركب بنسبة 10.4%، مدفوعًا ببرامج التشخيص الموسعة لطب الأطفال وفرض فحص حديثي الولادة.
- عن طريق الاختبار
بناءً على الاختبارات، يُقسّم سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) إلى اختبارات مخبرية واختبارات في نقطة الرعاية. وقد استحوذ قطاع الاختبارات المخبرية على الحصة الأكبر بنسبة 68.9% في عام 2024، مدعومًا ببنية تحتية مركزية للمختبرات وقدرة على معالجة كميات كبيرة.
من المتوقع أن ينمو قطاع اختبارات نقطة الرعاية (POCT) بأسرع معدل نمو سنوي مركب بنسبة 13.2% من عام 2025 إلى عام 2032، مما يعكس التحول نحو الاختبارات السريعة واللامركزية.
- حسب المستخدم النهائي
بناءً على المستخدم النهائي، يُقسّم سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) إلى مراكز تشخيص، ومستشفيات، ومعاهد أكاديمية وبحثية، ومراكز صحية مجتمعية، وعيادات، وخدمات رعاية صحية منزلية، وغيرها. وقد تصدر قطاع المستشفيات السوق بحصة بلغت 34.8% في عام 2024، بفضل قاعدة مرضاه الكبيرة وقدراته التشخيصية الداخلية.
من المتوقع أن ينمو قطاع الرعاية الصحية المنزلية بمعدل نمو سنوي مركب قدره 12.1% من عام 2025 إلى عام 2032، مدفوعًا بتفضيل المستهلكين للاختبار عن بعد وتوافر مجموعات تفاعل البوليميراز المتسلسل المحمولة.
تحليل إقليمي لسوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
- سيطرت أمريكا الشمالية على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) بحصة إيرادات بلغت 38.9% في عام 2024، مدفوعةً بالطلب المتزايد على أدوات التشخيص السريع، وازدياد انتشار الأمراض المعدية، والبنية التحتية الداعمة للرعاية الصحية. وقد أدى التركيز القوي للمنطقة على الكشف المبكر عن الأمراض، إلى جانب سياسات السداد المواتية، إلى تسريع اعتماد اختبارات تفاعل البوليميراز المتسلسل في مؤسسات الرعاية الصحية العامة والخاصة.
- يقدر مقدمو الرعاية الصحية والمرضى في المنطقة دقة وسرعة وتنوع تشخيصات تفاعل البوليميراز المتسلسل (PCR)، وخاصة في الكشف عن مسببات الأمراض مثل فيروس كورونا المستجد (SARS-CoV-2) والإنفلونزا وفيروس نقص المناعة البشرية وفيروس الورم الحليمي البشري.
- ويتم دعم هذا النمو أيضًا من خلال البنية التحتية المتقدمة للمختبرات، وارتفاع الإنفاق على الرعاية الصحية، والتعاون الاستراتيجي بين شركات التكنولوجيا الحيوية ومختبرات التشخيص، مما يضع التشخيص القائم على تفاعل البوليميراز المتسلسل (PCR) كعنصر أساسي في إدارة الأمراض المعدية في جميع أنحاء أمريكا الشمالية.
نظرة عامة على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) في الولايات المتحدة
استحوذ سوق الأمراض المعدية المعتمد على تفاعل البوليميراز المتسلسل (PCR) في الولايات المتحدة على أكبر حصة من الإيرادات، بنسبة 79% في عام 2024 في أمريكا الشمالية، مدفوعًا بتزايد المبادرات الحكومية للسيطرة على تفشي الأمراض المعدية وزيادة الوعي بالتشخيص الجزيئي. ولا تزال الولايات المتحدة رائدة في نشر اختبارات تفاعل البوليميراز المتسلسل في نقاط الرعاية، لا سيما في المستشفيات ومراكز الرعاية العاجلة ومختبرات التشخيص. كما يُسهم الاستخدام المتزايد للوحات تفاعل البوليميراز المتسلسل المتعددة وتوسع خدمات الرعاية الصحية عن بُعد بشكل كبير في نمو السوق. إضافةً إلى ذلك، يُسهم الحضور القوي لشركات رئيسية مثل Thermo Fisher Scientific وBD في دعم الابتكار والتوافر الواسع النطاق.
نظرة عامة على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) في أوروبا
من المتوقع أن يشهد سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) في أوروبا نموًا بمعدل نمو سنوي مركب كبير خلال الفترة المتوقعة، مدفوعًا بزيادة التركيز على مراقبة مقاومة مضادات الميكروبات، وبرامج الفحص الجزيئي المدعومة حكوميًا، والتأهب للأوبئة. ويعزز التحول المتزايد نحو الطب الشخصي والتشخيص المبكر استخدام تقنيات تفاعل البوليميراز المتسلسل في كل من المستشفيات العامة والمختبرات الخاصة. وتستثمر دول مثل ألمانيا وفرنسا والمملكة المتحدة في قدرات تفاعل البوليميراز المتسلسل كجزء من استراتيجياتها الصحية الوطنية.
نظرة عامة على سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) في المملكة المتحدة
من المتوقع أن ينمو سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) في المملكة المتحدة بمعدل نمو سنوي مركب ملحوظ، مدعومًا بدمج هيئة الخدمات الصحية الوطنية (NHS) للتشخيصات الجزيئية في برامج الفحص الروتينية. وقد أدى تزايد انتشار الأمراض المنقولة جنسيًا وأمراض الجهاز التنفسي إلى زيادة الطلب على اختبارات تفاعل البوليميراز المتسلسل السريعة في العيادات ومراكز الفحص المتنقلة. كما يلعب التمويل الحكومي المستمر لمراقبة متحورات كوفيد-19 وتتبع الأمراض الناشئة دورًا رئيسيًا في تطور السوق.
نظرة عامة على سوق الأمراض المعدية المعتمدة على تقنية تفاعل البوليميراز المتسلسل (PCR) في ألمانيا
من المتوقع أن يشهد سوق الأمراض المعدية المعتمدة على تقنية تفاعل البوليميراز المتسلسل (PCR) في ألمانيا نموًا ملحوظًا بمعدل نمو سنوي مركب، بفضل بنيته التحتية المتينة للرعاية الصحية ودعمه القوي لابتكارات التكنولوجيا الحيوية. ويستفيد السوق من الأتمتة السريعة للمختبرات، وتزايد أعداد كبار السن، وتزايد الطلب على حلول التشخيص عالية الحساسية. كما يُعتمد اختبار تفاعل البوليميراز المتسلسل على نطاق واسع في المستشفيات ومراكز التشخيص لإدارة العدوى المكتسبة من المستشفيات، وفحص مسببات الأمراض مثل المكورات العنقودية الذهبية المقاومة للميثيسيلين (MRSA) والمطثية العسيرة (C. difficile).
نظرة عامة على سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) في منطقة آسيا والمحيط الهادئ
من المتوقع أن ينمو سوق الأمراض المعدية المعتمدة على تفاعل البوليميراز المتسلسل (PCR) في منطقة آسيا والمحيط الهادئ بأسرع معدل نمو سنوي مركب قدره 11.3% بين عامي 2025 و2032، مدفوعًا بزيادة الاستثمارات في البنية التحتية للرعاية الصحية، ومبادرات مكافحة الأمراض المعدية التي تقودها الحكومات، وتزايد الوعي بالتشخيص المبكر في دول مثل الصين والهند واليابان. ويساهم الاستخدام الواسع النطاق لوسائل التشخيص المتنقلة، بالإضافة إلى السعي لتوفير مجموعات اختبار تفاعل البوليميراز المتسلسل بأسعار معقولة، في تسريع وتيرة انتشار هذه الخدمات في المناطق الريفية والحضرية على حد سواء.
نظرة عامة على سوق الأمراض المعدية المعتمدة على تقنية تفاعل البوليميراز المتسلسل (PCR) في اليابان
يشهد سوق الأمراض المعدية المعتمد على تفاعل البوليميراز المتسلسل (PCR) في اليابان نموًا مطردًا بفضل نظام الرعاية الصحية المتقدم تقنيًا وشيخوخة السكان. ويُعد الطلب المتزايد على إجراءات التشخيص منخفضة التدخل، وزيادة التمويل الحكومي لمراقبة الأمراض، ودمج تقنيات تفاعل البوليميراز المتسلسل اللحظي في التشخيصات الروتينية في المستشفيات، من العوامل الرئيسية الدافعة للنمو. ومن المتوقع أيضًا أن يُعزز تركيز اليابان على أتمتة المختبرات المدعومة بالذكاء الاصطناعي والرعاية الشخصية المزيد من التقدم في هذا القطاع.
نظرة عامة على سوق الأمراض المعدية المعتمدة على تقنية تفاعل البوليميراز المتسلسل (PCR) في الصين
استحوذ سوق الأمراض المعدية المعتمد على تفاعل البوليميراز المتسلسل (PCR) في الصين على أكبر حصة من الإيرادات في سوق الأمراض المعدية المعتمد على تفاعل البوليميراز المتسلسل في منطقة آسيا والمحيط الهادئ عام 2024، مدعومًا بتوسع الطبقة المتوسطة، وارتفاع معدلات الكشف عن العدوى، والاستثمارات الضخمة في البنية التحتية للصحة العامة. تُمكّن قاعدة التصنيع المحلية القوية في الصين من توفير مجموعات وأدوات تفاعل البوليميراز المتسلسل (PCR) فعّالة من حيث التكلفة، مما يُسهّل نشرها على نطاق واسع في المستشفيات والعيادات المجتمعية والمختبرات المتنقلة. ومن المتوقع أن يُسهم تركيز الحكومة على التأهب للأوبئة والأمن البيولوجي في دعم النمو في السنوات القادمة.
حصة سوق الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل
إن صناعة الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR) يقودها في المقام الأول شركات راسخة، بما في ذلك:
- مختبرات DNA الهند (الهند)
- دكتور سيف هاندز (الهند)
- مركز غانيش للتشخيص والتصوير الطبي (الهند)
- ماكس لاب (الهند)
- تشخيصات ميكروجين (الولايات المتحدة)
- باثلاب (الهند)
- عيادة واشنطن للسفر (الولايات المتحدة)
- عيادات كليرويل (المملكة المتحدة)
- أزوفا (الولايات المتحدة)
- مركز ون لايف للرعاية الصحية المنزلية (الهند)
- لال باث لابس (الهند)
أحدث التطورات في سوق الأمراض المعدية العالمية القائمة على تفاعل البوليميراز المتسلسل (PCR)
- في يوليو 2025، أطلقت شركة Seegene Inc. منصة STagora، وهي منصة جديدة تدمج بيانات التشخيص القائمة على تفاعل البوليميراز المتسلسل (PCR) مع النمذجة التنبؤية والتحليلات لتتبع الأمراض المعدية عالميًا في الوقت الفعلي. تدعم المنصة مراقبة الصحة العامة والاستجابة لتفشي الأمراض.
- في يونيو 2025، طرحت شركة Integrated DNA Technologies (IDT) طقم PrimeTime Influenza Kit ولوحات PrimeTime Research Pathogen Panels، المُحسّنة للكشف عن الإنفلونزا بتقنية qPCR وفحص مسببات الأمراض المتعددة. كُشف النقاب عن هذه الحلول في مؤتمر ASM Microbe 2025، مما يُتيح توسيع نطاق مراقبة الإنفلونزا ومسببات الأمراض وإجراء البحوث بشأنها.
- في يناير 2025، حصلت شركة بوش لحلول الرعاية الصحية على شهادة CE-IVDR لاختبار Vivalytic PCR، المصمم لتشخيص التهاب السحايا الجرثومي بسرعة في نقطة الرعاية. يُمكّن هذا الإنجاز التنظيمي من اعتماده سريريًا على نطاق أوسع في جميع أنحاء أوروبا.
- في يونيو 2025، أعادت شركة Qiagen تأكيد استراتيجيتها التي تستهدف تحقيق نمو سنوي في المبيعات بنسبة 7% تقريبًا حتى عام 2028، بالاعتماد على منصاتها لاختبارات الأمراض المعدية القائمة على تفاعل البوليميراز المتسلسل (PCR). وركزت الشركة على اختبارات حالات مثل التهاب السحايا والالتهاب الرئوي والسل والتهابات الجهاز الهضمي.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.