Global Electronic Clinical Outcome Assessment Ecoa Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
تجزئة سوق تقييم النتائج السريرية الإلكترونية العالمية (eCOA)، حسب النوع (تقييم النتائج المُبلّغ عنها من قِبل الطبيب (CLINRO)، وتقييم النتائج المُبلّغ عنها من قِبل المريض (PRO)، وتقييم النتائج المُبلّغ عنها من قِبل المراقب (OBSRO)، وتقييم نتائج الأداء (PERFO))، والوسيلة (حلول قائمة على الموقع، وحلول الويب، والأجهزة المحمولة)، والمستخدم النهائي (منظمات أبحاث العقود (CROs)، وشركات الأدوية والتكنولوجيا الحيوية، وشركات الأجهزة الطبية، والمستشفيات/مقدمي الرعاية الصحية، وشركات الخدمات الاستشارية، والمعاهد الأكاديمية والبحثية، وغيرها)، وطريقة التسليم (السحابية والمُستضاف على الويب) - اتجاهات الصناعة والتوقعات حتى عام 2032
حجم سوق تقييم النتائج السريرية الإلكترونية (eCOA)
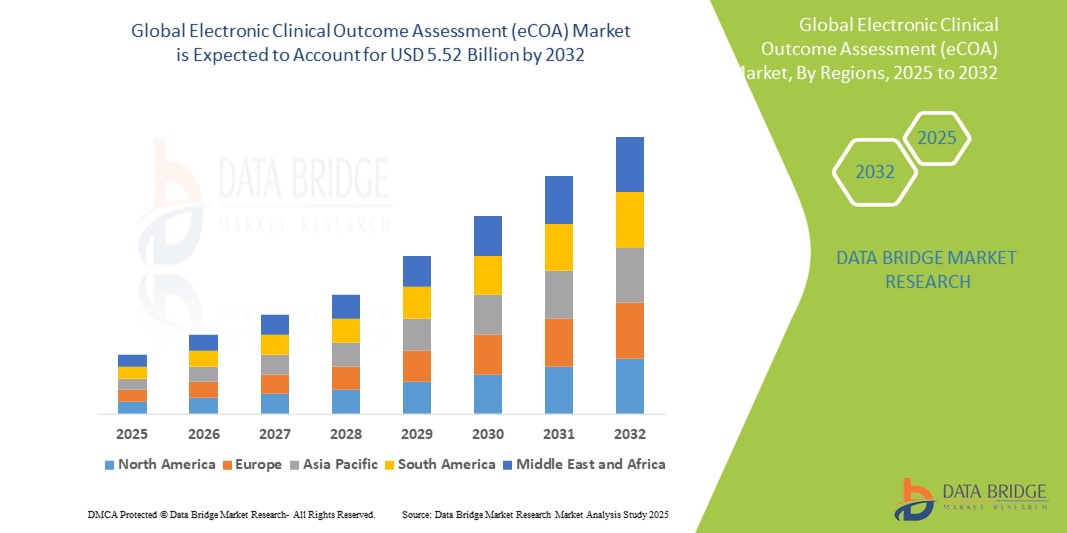
- تم تقييم حجم سوق التقييم الإلكتروني العالمي للنتائج السريرية (eCOA) بنحو 1.70 مليار دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 5.52 مليار دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 15.80٪ خلال الفترة المتوقعة.
- ينشأ نمو السوق في المقام الأول من خلال زيادة اعتماد التقنيات الرقمية في التجارب السريرية وأبحاث الرعاية الصحية، مما يسهل جمع بيانات المرضى ومراقبتها بشكل أكثر دقة وكفاءة
- بالإضافة إلى ذلك، فإن الطلب المتزايد على رؤى المرضى في الوقت الفعلي، والامتثال التنظيمي المحسن، وتعزيز سلامة البيانات يدفع إلى زيادة استخدام حلول eCOA عبر شركات الأدوية، ومنظمات أبحاث العقود (CROs)، ومقدمي الرعاية الصحية
تحليل سوق تقييم النتائج السريرية الإلكترونية (eCOA)
- حلول eCOA، التي تتيح التقاط بيانات النتائج السريرية إلكترونيًا مباشرةً من المرضى أو مقدمي الرعاية أو الأطباء، أصبحت مكونات حيوية بشكل متزايد للتجارب السريرية الحديثة وأبحاث الرعاية الصحية نظرًا لدقة البيانات المحسنة وقدرات المراقبة في الوقت الفعلي والتكامل السلس مع النظم البيئية الصحية الرقمية.
- الطلب المتزايد على eCOA مدفوع في المقام الأول بالاعتماد الواسع النطاق لتقنيات الصحة الرقمية، والتركيز المتزايد على التجارب التي تركز على المريض، والتفضيل المتزايد لطرق جمع البيانات عن بعد وسهلة الاستخدام والتي تعمل على تحسين كفاءة التجارب والامتثال.
- تهيمن أمريكا الشمالية على سوق تقييم النتائج السريرية الإلكترونية (eCOA) بأكبر حصة إيرادات بنسبة 43.5٪ في عام 2024، وتتميز بالتبني المبكر لحلول التجارب السريرية الرقمية، وقطاعات الأدوية والتكنولوجيا الحيوية القوية، والأطر التنظيمية التي تدعم التقاط البيانات الإلكترونية، مع الولايات المتحدة التي تشهد نموًا كبيرًا مدفوعًا بالابتكارات من كل من البائعين الراسخين ومقدمي التكنولوجيا الناشئة الذين يركزون على المنصات المحمولة والمستندة إلى السحابة.
- من المتوقع أن تكون منطقة آسيا والمحيط الهادئ أسرع المناطق نموًا في سوق تقييم النتائج السريرية الإلكترونية (eCOA) خلال الفترة المتوقعة بسبب زيادة أنشطة التجارب السريرية، وارتفاع استثمارات الرعاية الصحية، والوعي المتزايد بفوائد الأدوات الرقمية في الأسواق الناشئة مثل الصين والهند.
- تهيمن شريحة تقييم النتائج المبلغ عنها للمريض (PRO) على سوق تقييم النتائج السريرية الإلكترونية (eCOA) بحصة سوقية تبلغ 48.5% في عام 2024، مدفوعًا بدورها الحاسم في التقاط وجهات نظر المرضى حول فعالية العلاج وجودة الحياة، والتي أصبحت ذات أولوية متزايدة من قبل الرعاة والهيئات التنظيمية.
نطاق التقرير وتجزئة سوق تقييم النتائج السريرية الإلكترونية (eCOA)
|
صفات |
رؤى السوق الرئيسية لتقييم النتائج السريرية الإلكترونية (eCOA) |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
أمريكا الشمالية
أوروبا
آسيا والمحيط الهادئ
الشرق الأوسط وأفريقيا
أمريكا الجنوبية
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تشمل أيضًا تحليلًا متعمقًا من الخبراء، وتحليل التسعير، وتحليل حصة العلامة التجارية، واستطلاع رأي المستهلكين، وتحليل التركيبة السكانية، وتحليل سلسلة التوريد، وتحليل سلسلة القيمة، ونظرة عامة على المواد الخام / المواد الاستهلاكية، ومعايير اختيار البائعين، وتحليل PESTLE، وتحليل Porter، والإطار التنظيمي. |
اتجاهات سوق تقييم النتائج السريرية الإلكترونية (eCOA)
"تحسين كفاءة التجارب السريرية من خلال الذكاء الاصطناعي ومراقبة المرضى عن بُعد"
- من الاتجاهات المهمة والمتسارعة في سوق eCOA العالمي التكامل المتزايد لتقنيات الذكاء الاصطناعي ومراقبة المرضى عن بُعد ضمن منصات جمع بيانات التجارب السريرية. يُحسّن هذا التكامل التقني بشكل كبير دقة تقييمات النتائج السريرية وتوقيتها وتركيزها على المرضى.
- على سبيل المثال، يُدمج مُقدّمو eCOA الرائدون، مثل Medidata وERT، تحليلاتٍ قائمةً على الذكاء الاصطناعي لتحديد الأنماط في بيانات المرضى المُبلّغ عنها، مما يُتيح الكشف المُبكر عن الآثار الجانبية وتحسين عملية اتخاذ القرارات بشأن التجارب السريرية. وبالمثل، تُسهّل الأجهزة القابلة للارتداء المُقترنة بمنصات eCOA المراقبة المُستمرة والفورية لمقاييس صحة المرضى، بما يتجاوز الزيارات الميدانية التقليدية.
- يُتيح دمج الذكاء الاصطناعي في eCOA ميزاتٍ مثل التحليلات التنبؤية لالتزام المرضى بالعلاج، وفحوصات جودة البيانات الآلية، والتنبيهات الذكية للاستجابات غير الاعتيادية للمرضى. علاوةً على ذلك، تُوفر إمكانيات المراقبة عن بُعد للمرضى واجهات استخدام مريحة وسهلة للإبلاغ عن النتائج من المنزل، مما يُحسّن اكتمال البيانات وتفاعل المرضى.
- يتيح التكامل السلس لأنظمة eCOA مع منصات إدارة الصحة الرقمية والتجارب السريرية الأوسع نطاقًا للجهات الراعية مركزية إدارة البيانات وتبسيط سير عمل التجارب. ومن خلال لوحات معلومات موحدة، يمكن للفرق السريرية مراقبة بيانات المرضى وأداء الموقع والامتثال للوائح التنظيمية في الوقت الفعلي.
- هذا التوجه نحو حلول نتائج سريرية أكثر ذكاءً وترابطًا وسهولةً للمرضى يُعيد صياغة توقعات جمع بيانات التجارب السريرية بشكل جذري. ونتيجةً لذلك، تعمل شركات مثل Oracle Health وCRF Health على تطوير منصات eCOA مُدعّمة بالذكاء الاصطناعي، مع قدرات تنبؤية مُحسّنة ووظائف جمع بيانات عن بُعد.
- يتزايد الطلب على حلول eCOA التي تتميز بتكامل الذكاء الاصطناعي ومراقبة المرضى عن بعد بسرعة في قطاعات الأدوية والتكنولوجيا الحيوية والأجهزة الطبية، حيث يعطي أصحاب المصلحة الأولوية بشكل متزايد لكفاءة التجارب ودقة البيانات وتجربة المريض
ديناميكيات سوق تقييم النتائج السريرية الإلكترونية (eCOA)
سائق
"تزايد الطلب على التجارب التي تركز على المرضى ودقة البيانات الرقمية"
- إن التركيز المتزايد على التجارب السريرية التي تركز على المريض، إلى جانب الحاجة المتزايدة إلى جمع بيانات رقمية دقيقة في الوقت الفعلي، يعد محركًا مهمًا للطلب المتزايد على حلول تقييم النتائج السريرية الإلكترونية (eCOA).
- على سبيل المثال، في يناير 2024، قدمت شركة Medidata، التابعة لشركة Dassault Systèmes، تحسينات جديدة مدعومة بالذكاء الاصطناعي على منصة eCOA الخاصة بها لتحسين امتثال المرضى وجودة البيانات في التجارب اللامركزية. ومن المتوقع أن تدفع هذه الابتكارات من قِبل الجهات الفاعلة الرئيسية في هذا القطاع نمو سوق eCOA خلال الفترة المتوقعة.
- مع سعي شركات الأدوية والتكنولوجيا الحيوية إلى تبسيط عمليات التجارب السريرية وتقليل الوقت اللازم لطرح المنتجات في السوق، توفر منصات eCOA ميزات متقدمة مثل التقاط البيانات في الوقت الفعلي، وإعداد التقارير عن المرضى عن بعد، والتحقق الآلي، مما يوفر تحسنًا كبيرًا على الطرق التقليدية القائمة على الورق.
- علاوة على ذلك، فإن التبني المتزايد لنماذج التجارب السريرية اللامركزية والهجينة يضع eCOA كمكون أساسي لجمع البيانات عن بعد، مما يحسن مشاركة المرضى مع الحفاظ على معايير عالية من الامتثال التنظيمي.
- تُعدّ قدرة منصات eCOA على تعزيز كفاءة التجارب من خلال جمع البيانات إلكترونيًا، ودعم اللغات المتعددة، والتكامل مع الأجهزة القابلة للارتداء أو تطبيقات الهاتف المحمول عاملًا رئيسيًا في تعزيز اعتمادها لدى منظمات البحوث السريرية وشركات الأدوية ومؤسسات البحث. كما أن التركيز المتزايد على خفض معدلات الانسحاب من التجارب السريرية وتحسين سلامة البيانات يدعم التكامل الواسع لحلول eCOA في الأبحاث السريرية الحديثة.
ضبط النفس/التحدي
"المخاوف بشأن خصوصية البيانات والامتثال التنظيمي وتكاليف التنفيذ المرتفعة"
- تشكل المخاوف المحيطة بخصوصية البيانات والامتثال التنظيمي وتكاليف التنفيذ الأولية المرتفعة لمنصات تقييم النتائج السريرية الإلكترونية (eCOA) تحديات كبيرة أمام تبني السوق الأوسع
- نظرًا لأن أنظمة eCOA تتضمن التقاط ونقل بيانات صحية حساسة للمرضى إلكترونيًا، فهي تخضع لأنظمة صارمة لحماية البيانات مثل HIPAA وGDPR و21 CFR الجزء 11، مما يجعل الامتثال معقدًا ويتطلب موارد كثيرة للرعاة ومنظمات البحث السريرية
- على سبيل المثال، أعرب العديد من رعاة التجارب السريرية عن حذرهم في التحول الكامل إلى أنظمة eCOA بسبب عدم اليقين بشأن قواعد توطين البيانات وتعقيد ضمان الامتثال للبيانات عبر الحدود، وخاصة في التجارب متعددة المناطق.
- يتطلب التصدي لهذه التحديات بنية تحتية قوية لأمن البيانات، وعمليات تدقيق دورية، والالتزام بمعايير الامتثال العالمية. يستثمر مزودو شهادات الاعتماد الإلكترونية (eCOA) الرائدون، مثل Oracle Health وSignant Health، بشكل كبير في المنصات المشفرة والتدريب التنظيمي للحد من المخاطر والحفاظ على ثقة الجهات المعنية بالتجارب.
- بالإضافة إلى ذلك، قد تُشكّل التكلفة الأولية المرتفعة المرتبطة بنشر أنظمة eCOA - بما في ذلك رسوم الترخيص، وشراء الأجهزة، وتدريب الموظفين، وتكامل النظام - عائقًا أمام دخول السوق، لا سيما بالنسبة لمؤسسات البحث الصغيرة والمتوسطة. على الرغم من الاعتراف الواسع بالفوائد طويلة الأجل، مثل تحسين دقة البيانات وتقليل مدة التجارب، إلا أن العبء المالي الأولي قد يحدّ من اعتماد هذه الأنظمة في المؤسسات محدودة الموارد.
- سيكون التغلب على هذه التحديات من خلال نماذج التسعير القابلة للتطوير والتسليم المستند إلى السحابة والابتكار المستمر في المنصات الآمنة وسهلة الاستخدام أمرًا ضروريًا لدفع التبني الأوسع والمستدام لحلول eCOA في جميع أنحاء المشهد البحثي السريري.
نطاق سوق تقييم النتائج السريرية الإلكترونية (eCOA)
يتم تقسيم السوق على أساس النوع والوسيلة والمستخدم النهائي وطريقة التسليم
- حسب النوع
يُقسّم سوق تقييم النتائج السريرية الإلكترونية (eCOA) حسب نوعه إلى: النتائج المُبلّغ عنها من قِبل المرضى (PRO)، والنتائج المُبلّغ عنها من قِبل الأطباء (ClinRO)، والنتائج المُبلّغ عنها من قِبل المراقب (ObsRO)، ونتائج الأداء (PerfO). وقد استحوذ قطاع النتائج المُبلّغ عنها من قِبل المرضى (PRO) على أكبر حصة من إيرادات السوق بنسبة 48.5% في عام 2024، بفضل نهجه المُركّز على المرضى في جمع رؤى مباشرة حول تجاربهم وأعراضهم ونتائج علاجهم. تُمكّن أدوات التقييم المرضى من الإبلاغ مباشرةً عن بياناتهم الصحية آنيًا عبر منصات إلكترونية، مما يُحسّن دقة البيانات ويزيد من مشاركة المرضى، مما يُحسّن جودة الدراسات السريرية في نهاية المطاف.
من المتوقع أن يشهد قطاع النتائج المُبلّغ عنها سريريًا (ClinRO) نموًا كبيرًا خلال فترة التوقعات، نظرًا لتزايد تعقيد التجارب السريرية والحاجة إلى أساليب دقيقة وموحدة لجمع البيانات. يتضمن ClinRO تقييمات يُجريها متخصصون مُدرّبون في مجال الرعاية الصحية، مما يُوفر بيانات موضوعية وموثوقة لتقييم التدخلات السريرية، لا سيما في الحالات التي يتعذر فيها الإبلاغ الذاتي من قِبَل المريض.
- حسب النمط
بناءً على طريقة الاستخدام، يُقسّم سوق تقييم النتائج السريرية الإلكتروني (eCOA) إلى حلول قائمة على الموقع، وحلول ويب، وأجهزة محمولة. وقد حقق قطاع حلول الويب أكبر حصة من إيرادات السوق في عام 2024، بفضل واجهاته سهلة الاستخدام، وسهولة الوصول إليه، وانخفاض احتياجاته الاستثمارية. تُخزّن الحلول المُستضافة على الويب بيانات العملاء على خوادم سحابية، يمكن الوصول إليها عبر الويب باستخدام أجهزة حاسوب أساسية واتصال بالإنترنت، مما يوفر مرونة في التخصيص ويُمكّن من تصميمها بما يُلبي احتياجات العملاء المحددة.
من المتوقع أن يشهد قطاع الأجهزة المحمولة نموًا ملحوظًا خلال فترة التوقعات، مدفوعًا بالاعتماد المتزايد على تقنيات الهاتف المحمول في التجارب السريرية. تُسهّل هذه الأجهزة التقاط البيانات آنيًا وتُحسّن التزام المرضى بالعلاج، مما يجعلها خيارًا جذابًا للدراسات السريرية اللامركزية والبعيدة.
- حسب المستخدم النهائي
بناءً على المستخدم النهائي، يُقسّم سوق التقييم الإلكتروني للنتائج السريرية (eCOA) إلى شركات الأدوية والتكنولوجيا الحيوية، ومنظمات البحوث التعاقدية (CROs)، وشركات الأجهزة الطبية، والمستشفيات/مقدمي الرعاية الصحية، وشركات الخدمات الاستشارية، والمعاهد الأكاديمية والبحثية، وغيرها. يهيمن قطاع شركات الأدوية والتكنولوجيا الحيوية على السوق، حيث سيستحوذ على 50.66% من حصة السوق في عام 2024. وتعود هذه الهيمنة إلى الدور المحوري الذي تلعبه حلول eCOA في تبسيط جمع البيانات وتحليلها خلال عمليات تطوير الأدوية، وضمان الامتثال للمعايير التنظيمية، وتعزيز دقة بيانات التجارب السريرية.
من المتوقع أن يشهد قطاع منظمات الأبحاث التعاقدية (CROs) نموًا ملحوظًا خلال فترة التوقعات، مدفوعًا بتوجه شركات الأدوية الحيوية والأجهزة الطبية الكبرى نحو الاستعانة بمصادر خارجية لإدارة الأبحاث السريرية. تقدم منظمات الأبحاث التعاقدية خدمات شاملة تشمل تصميم الدراسات، وتجنيد المرضى، وجمع البيانات، وتحليلها، مما يجعلها لاعبًا أساسيًا في مجال eCOA.
حسب طريقة التسليم
بناءً على طريقة التسليم، يُقسّم سوق تقييم النتائج السريرية الإلكتروني (eCOA) إلى حلول سحابية وحلول مُستضافة على الويب. سيستحوذ قطاع الحلول المُستضافة على الويب على أكبر حصة سوقية بنسبة 58.9% بحلول عام 2025، نظرًا لفعاليته من حيث التكلفة مقارنةً بالحلول السحابية. تتطلب المنصات المُستضافة على الويب استثمارات أولية أقل في البنية التحتية للمستخدمين النهائيين، مما يُقلل النفقات الرأسمالية لشركات الأدوية، ومنظمات البحث التعاقدي، ومقدمي الرعاية الصحية.
من المتوقع أن يشهد قطاع الحلول السحابية نموًا ملحوظًا خلال فترة التوقعات، بفضل قابليته للتوسع ومرونته وفعاليته من حيث التكلفة. تُسهّل المنصات السحابية وصول أصحاب المصلحة في التجارب السريرية إلى البيانات بشكل أسهل وأسرع، بغض النظر عن موقعهم، وهو أمر بالغ الأهمية للتجارب متعددة المواقع.
تحليل إقليمي لسوق تقييم النتائج السريرية الإلكترونية (eCOA)
- تهيمن أمريكا الشمالية على سوق تقييم النتائج السريرية الإلكترونية (eCOA) بأكبر حصة إيرادات بنسبة 43.5٪ في عام 2024، مدفوعة بالتبني المبكر لحلول التجارب السريرية الرقمية، وقطاعات الأدوية والتكنولوجيا الحيوية القوية، والأطر التنظيمية التي تدعم التقاط البيانات الإلكترونية.
- تستفيد المنطقة من إطار تنظيمي قوي يدعم التحول الرقمي للأبحاث السريرية، مما يشجع شركات الأدوية ومنظمات الأبحاث التعاقدية على اعتماد منصات eCOA لتعزيز دقة البيانات والامتثال التنظيمي
- بالإضافة إلى ذلك، تُسهم الاستثمارات القوية في البحث والتطوير، والبنية التحتية الراسخة للرعاية الصحية، والاعتماد المُبكر للتجارب السريرية اللامركزية والمُركزة على المرضى، بشكل كبير في نمو السوق. كما يُسرّع وجود مُزوّدي حلول eCOA الرائدين ومنظمات البحث التعاقدي (CROs) التوسع الإقليمي لأدوات تقييم النتائج السريرية الإلكترونية.
نظرة عامة على سوق التقييم الإلكتروني للنتائج السريرية (eCOA) في الولايات المتحدة
استحوذ سوق التقييم الإلكتروني للنتائج السريرية (eCOA) في الولايات المتحدة على أكبر حصة من الإيرادات، بنسبة 79.6% في عام 2024، في أمريكا الشمالية، مدفوعًا بريادة البلاد في التجارب السريرية والرقمنة السريعة لممارسات البحث السريري. وتدعو الهيئات التنظيمية، مثل إدارة الغذاء والدواء الأمريكية (FDA)، بقوة إلى استخدام الأدوات الرقمية لتحسين جودة البيانات وإشراك المرضى، مما يساهم في انتشار استخدام أنظمة eCOA على نطاق واسع. إضافةً إلى ذلك، تُغذي الحاجة المتزايدة إلى نماذج التجارب السريرية اللامركزية والهجينة الطلب على منصات جمع بيانات المرضى عن بُعد وفي الوقت الفعلي. كما يستفيد السوق الأمريكي من التمويل القوي للبحث والتطوير، والتواجد القوي لشركات الأدوية العملاقة، والبنية التحتية المتطورة لتكنولوجيا المعلومات الصحية.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية في أوروبا (eCOA)
من المتوقع أن يشهد سوق تقييم النتائج السريرية الإلكترونية (eCOA) في أوروبا نموًا بمعدل نمو سنوي مركب كبير خلال فترة التوقعات، مدفوعًا بتركيز الجهات التنظيمية المتزايد على الأدلة العملية، والتركيز على المرضى، وتوحيد البيانات في التجارب السريرية. وتُسرّع الحاجة المتزايدة إلى حلول رقمية متعددة اللغات ومُكيّفة ثقافيًا في جميع أنحاء الاتحاد الأوروبي من اعتماد منصات eCOA مرنة وقابلة للتطوير. علاوة على ذلك، يدعم ازدياد التعاون البحثي الأكاديمي، إلى جانب السياسات المواتية للتحول الرقمي في مجال الصحة، النمو الإقليمي. وتُعد دول مثل ألمانيا والمملكة المتحدة وفرنسا رائدة في تبني التكنولوجيا ضمن منظوماتها البيئية للتجارب السريرية.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية (eCOA) في المملكة المتحدة
من المتوقع أن ينمو سوق تقييم النتائج السريرية الإلكترونية (eCOA) في المملكة المتحدة بمعدل نمو سنوي مركب ملحوظ خلال فترة التوقعات، مدعومًا بقوة قطاع البحث والتطوير في مجال الأدوية الحيوية واستراتيجيات الصحة الرقمية الاستباقية التي تتبناها هيئة الخدمات الصحية الوطنية. ويعزز العدد المتزايد من التجارب اللامركزية والوضوح التنظيمي المتعلق بجمع البيانات إلكترونيًا من تبني السوق. ومع وجود بيئة بحثية سريرية متقدمة واستثمارات كبيرة في المعلوماتية الصحية، تشهد المملكة المتحدة إقبالًا سريعًا على تقنيات eCOA لضمان الامتثال، وتحسين مشاركة المرضى، وتمكين تتبع النتائج بكفاءة.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية (eCOA) في ألمانيا
من المتوقع أن يشهد سوق تقييم النتائج السريرية الإلكترونية (eCOA) في ألمانيا نموًا ملحوظًا بمعدل نمو سنوي مركب خلال الفترة المتوقعة، مدفوعًا بسمعة البلاد المتميزة في التجارب السريرية وقوانين حماية البيانات الصارمة. تُشدد الهيئات التنظيمية الألمانية على موثوقية وأمان البيانات السريرية، مما يدفع الجهات الراعية ومنظمات البحوث السريرية إلى الاستثمار في حلول eCOA آمنة ومعتمدة. إضافةً إلى ذلك، يُعزز الطلب المتزايد في ألمانيا على التقاط البيانات آنيًا في التجارب السريرية من المرحلة الأولى إلى الرابعة، وبنيتها التحتية القوية لتكنولوجيا المعلومات في مجال الرعاية الصحية، تكامل تقنيات eCOA بشكل أكبر في أبحاث الأجهزة الطبية والأدوية.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية (eCOA) في منطقة آسيا والمحيط الهادئ
من المتوقع أن يشهد سوق تقييم النتائج السريرية الإلكترونية (eCOA) في منطقة آسيا والمحيط الهادئ نموًا بمعدل نمو سنوي مركب (CAGR) أسرع خلال الفترة المتوقعة من 2025 إلى 2032، مدفوعًا بتزايد أنشطة البحث السريري والتحول الرقمي للرعاية الصحية في دول مثل الصين والهند وكوريا الجنوبية واليابان. يدعم التوسع في التجارب السريرية متعددة الجنسيات وتوافر فئات متنوعة من المرضى النمو الإقليمي. كما أن الحوافز الحكومية لاعتماد منصات الصحة الرقمية والطلب المتزايد على الحلول القائمة على الهواتف المحمولة تجعل اعتماد eCOA أكثر جدوى وانتشارًا في المناطق الحضرية وشبه الحضرية. كما تُسهم الشراكات المحلية بين منظمات البحث التعاقدي وشركات الأدوية العالمية في تعزيز تطبيق eCOA.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية (eCOA) في اليابان
يشهد سوق تقييم النتائج السريرية الإلكترونية (eCOA) في اليابان زخمًا متزايدًا بفضل التطور التكنولوجي في البلاد، وشيخوخة السكان، والتركيز على جودة البيانات في التجارب السريرية. وتبدي الهيئة التنظيمية اليابانية، PMDA، تقبّلًا متزايدًا لنقاط النهاية الرقمية وأدوات التقاط البيانات عن بُعد. كما يشهد السوق تغيّرًا ملحوظًا بفضل زيادة التجارب السريرية المنزلية والخارجية، مما يزيد من الحاجة إلى أنظمة eCOA دقيقة وسهلة الاستخدام للمرضى. ومن المتوقع أن يُسهم التكامل مع منظومات eClinical الأوسع وأدوات إشراك المرضى المدعومة بالذكاء الاصطناعي في تسريع النمو.
نظرة عامة على سوق تقييم النتائج السريرية الإلكترونية (eCOA) في الهند
استحوذ سوق التقييم الإلكتروني للنتائج السريرية (eCOA) في الهند على أكبر حصة من إيرادات السوق في منطقة آسيا والمحيط الهادئ عام 2024، مدفوعًا بالزيادة الكبيرة في نشاط التجارب السريرية، ووجود شريحة سكانية متمرسة في مجال التكنولوجيا، وتوسع قدرات تصنيع الأدوية. وتشجع بيئة منظمات البحث التعاقدي (CRO) الفعالة من حيث التكلفة في الهند، والسياسات الحكومية الداعمة لرقمنة الرعاية الصحية، الجهات الراعية العالمية على نشر أدوات eCOA في التجارب المحلية. كما أن تزايد انتشار الهواتف الذكية، ونمو التطبيب عن بُعد، وتحسين اتصال الإنترنت في المناطق الحضرية وشبه الحضرية، يجعل منصات eCOA القائمة على الهواتف المحمولة والمستضافة على السحابة أكثر سهولة في الوصول إليها وانتشارًا واسعًا.
حصة سوق تقييم النتائج السريرية الإلكترونية (eCOA)
إن صناعة تقييم النتائج السريرية الإلكترونية (eCOA) يقودها في المقام الأول شركات راسخة، بما في ذلك:
- إيكويا (الولايات المتحدة)
- كلاريو (الولايات المتحدة)
- ميديداتا (الولايات المتحدة)
- أنظمة فيفا (الولايات المتحدة)
- تكنولوجيا موارد الأرض (الولايات المتحدة)
- أوراكل للعلوم الصحية (الولايات المتحدة)
- YPrime, LLC (الولايات المتحدة)
- شركة أريس جلوبال ذ.م.م (الولايات المتحدة)
- كاستور EDC (هولندا)
- eClinicalWorks (الولايات المتحدة)
- شركة ميدريو (الولايات المتحدة)
- كلين ون (الولايات المتحدة)
- سيجنانت هيلث (الولايات المتحدة)
- شركة كلينيكال إنك (الولايات المتحدة)
- شركة Curebase, Inc. (الولايات المتحدة)
- كايينتيس (فرنسا)
- كاليكس (المملكة المتحدة)
- صحة مكعب البيانات (الولايات المتحدة)
- HealthDiary, Inc. (الولايات المتحدة)
أحدث التطورات في سوق تقييم النتائج السريرية الإلكترونية العالمية (eCOA)
- في مايو 2025، استحوذت شركة كلاريو (الولايات المتحدة) على أعمال eCOA التابعة لشركة WCG Clinical (الولايات المتحدة)، في خطوة استراتيجية لتعزيز ريادتها في مجال حلول بيانات نقاط النهاية الرقمية، وخاصةً للتجارب السريرية في مجال علم الأعصاب. يُوسّع هذا الاستحواذ منصة بيانات نقاط النهاية الشاملة لشركة كلاريو، مما يُتيح دعمًا أفضل لبيئات التجارب المعقدة، ويُعزز مكانتها في مجال eCOA سريع التطور.
- في مايو 2025، واصل معهد المسار الحرج (الولايات المتحدة) مبادرته "eCOA: معًا نحو الأفضل"، والتي تهدف إلى توحيد الجهات الراعية وموردي التكنولوجيا والجهات التنظيمية. يركز هذا الجهد التعاوني، الممتد حتى مارس 2025، على إرساء أفضل الممارسات قبل المنافسة ووضع قاموس مشترك لجمع بيانات eCOA، وتعزيز التوحيد القياسي وتسريع اعتماده في مختلف المناطق.
- في نوفمبر 2023، عززت شركة كلينيكال إنك مجموعة خدماتها لإشراك المرضى من خلال دمج أداة التشخيص السلوكي SPUR من شركة Observia. يدمج هذا التكامل التقييم السلوكي مع تعديل نمط الحياة، وeCOA، وeSource، والمؤشرات الحيوية الرقمية، بهدف توفير فهم أكثر شمولية لسلوك المرضى وتحسين نتائج التجارب.
- في أكتوبر 2023، دخلت شركة كلاريو في شراكة استراتيجية مع شركة ترايل داتا، وهي شركة تقدم خدمات التجارب السريرية اللامركزية. يُعزز هذا التعاون حضور كلاريو في مجال التجارب السريرية في الصين، حيث يجمع خبرتهما لتقديم حلول لامركزية متطورة للتجارب السريرية، وتطوير مناهج تركز على المرضى في المنطقة.
- في ديسمبر 2022، أطلقت شركة Suvoda LLC، وهي شركة متخصصة في تكنولوجيا التجارب السريرية eCOA، مجموعة أدوات تصميم تقييمات النتائج السريرية الإلكترونية (eCOA). صُممت هذه المجموعة لتتكامل بسلاسة مع Suvoda IRT وeConsent، مما يُعالج أوجه القصور التاريخية في تطبيق eCOA، ويهدف إلى تبسيط عملية التصميم.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.