Europe Anti Nuclear Antibody Test Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
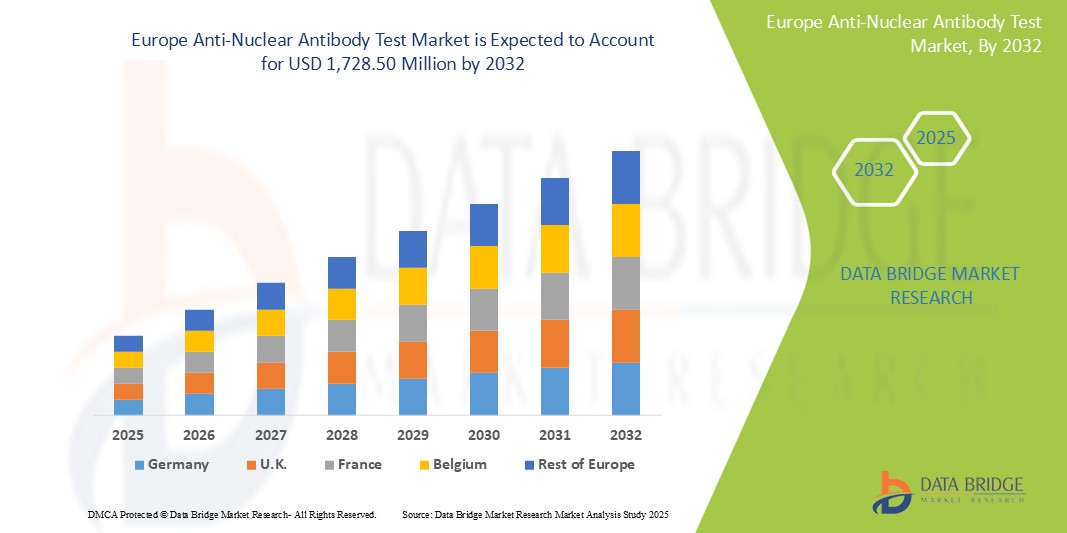
659.47 Million
USD
1,728.50 Million
2024
2032
USD
659.47 Million
USD
1,728.50 Million
2024
2032
| 2025 –2032 | |
| USD 659.47 Million | |
| USD 1,728.50 Million | |
|
|
|
|
تجزئة سوق اختبارات الأجسام المضادة النووية في أوروبا، حسب نوع الجسم المضاد (مستضدات نووية مستخلصة (ENA)، ومضادات DSDNA والهستونات، ومضادات DFS70، ومضادات PM-SCL، ومضادات السنترومير، ومضادات SP100، وغيرها)، والمنتج (الأجهزة، والمواد الاستهلاكية، والكواشف، والخدمات)، والتقنية (ELISA، والفلورة المناعية غير المباشرة (IIF)، واختبارات النشاف، ومصفوفات المستضدات الدقيقة، والتقنيات القائمة على الهلام، والاختبار المتعدد، وقياس التدفق الخلوي، والتراص الدموي السلبي (PHA)، وغيرها)، والتطبيق (أمراض المناعة الذاتية والأمراض المعدية)، والمستخدم النهائي (المستشفيات، والمختبرات، ومراكز التشخيص، ومعاهد البحث، وغيرها)، وقنوات التوزيع (العطاء المباشر، ومبيعات التجزئة، والموزعون الخارجيون، وغيرها) - اتجاهات الصناعة وتوقعاتها حتى عام 2032
حجم سوق اختبار الأجسام المضادة النووية في أوروبا
- تم تقييم حجم سوق اختبار الأجسام المضادة النووية في أوروبا بـ 659.47 مليون دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 1،728.50 مليون دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 12.8٪ خلال الفترة المتوقعة
- ويعود هذا النمو إلى عوامل مثل الانتشار المتزايد لأمراض المناعة الذاتية، والتقدم التكنولوجي في أساليب التشخيص، وزيادة الوعي العام ومبادرات الكشف المبكر.
تحليل سوق اختبار الأجسام المضادة النووية في أوروبا
- تُعدّ اختبارات الأجسام المضادة للنواة (ANA) أدوات تشخيصية بالغة الأهمية تُستخدم للكشف عن الأجسام المضادة الذاتية في الدم، مما يُساعد في تشخيص أمراض المناعة الذاتية مثل الذئبة الحمامية الجهازية (SLE)، والتهاب المفاصل الروماتويدي، ومتلازمة شوغرن. وتلعب هذه الاختبارات دورًا حيويًا في الكشف المبكر عن هذه الحالات وعلاجها.
- إن الطلب على اختبارات ANA مدفوع بشكل كبير بالانتشار المتزايد للاضطرابات المناعية الذاتية، وزيادة الوعي بالتشخيص المبكر، والتقدم في تقنيات التشخيص
- من المتوقع أن تهيمن ألمانيا على سوق اختبار الأجسام المضادة النووية في أوروبا بحصة سوقية تبلغ 25.6٪ بسبب نظام الرعاية الصحية المتقدم لديها والوعي العالي للمرضى ووجود تقنيات الاختبار المتقدمة
- من المتوقع أن تكون إيطاليا أسرع الدول نموًا في سوق اختبارات الأجسام المضادة النووية في أوروبا، بمعدل نمو سنوي مركب قدره 13.4%، وذلك نتيجةً لزيادة أمراض المناعة الذاتية مثل الذئبة، والتهاب المفاصل الروماتويدي، ومتلازمة شوغرن. وقد أدى هذا العبء المرضي المتزايد إلى زيادة الطلب على الاختبارات التشخيصية المبكرة والدقيقة، بما في ذلك اختبارات الأجسام المضادة للنواة (ANA).
- من المتوقع أن تهيمن تقنية المناعة الفلورية غير المباشرة (IIF) على السوق بحصة سوقية تبلغ 59.9%. تُعزى هذه الهيمنة إلى مكانتها كمعيار ذهبي لاختبارات الأجسام المضادة الذاتية (ANA)، حيث توفر حساسية عالية وقدرة على اكتشاف مجموعة واسعة من الأجسام المضادة الذاتية.
نطاق التقرير وتجزئة سوق اختبار الأجسام المضادة النووية في أوروبا
|
صفات |
رؤى رئيسية حول سوق اختبار الأجسام المضادة النووية في أوروبا |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
أوروبا
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، تتضمن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research أيضًا تحليل الاستيراد والتصدير، ونظرة عامة على القدرة الإنتاجية، وتحليل استهلاك الإنتاج، وتحليل اتجاه الأسعار، وسيناريو تغير المناخ، وتحليل سلسلة التوريد، وتحليل سلسلة القيمة، ونظرة عامة على المواد الخام / المواد الاستهلاكية، ومعايير اختيار البائعين، وتحليل PESTLE، وتحليل بورتر، والإطار التنظيمي. |
اتجاهات سوق اختبار الأجسام المضادة النووية في أوروبا
"التطورات التكنولوجية في اختبار الأجسام المضادة النووية لتشخيص أمراض المناعة الذاتية"
- أحد الاتجاهات البارزة في سوق اختبار الأجسام المضادة النووية في أوروبا هو التكامل المتزايد لتقنيات التشخيص المتقدمة، بما في ذلك الأنظمة الآلية والاختبارات المتعددة، لتحسين الدقة والكفاءة
- تعمل هذه الابتكارات على تعزيز دقة التشخيص من خلال السماح بالكشف المتزامن عن العديد من الأجسام المضادة الذاتية، مما يقلل من أوقات الاستجابة ويقلل من الخطأ البشري، وبالتالي دعم التشخيص المبكر والعلاج الشخصي.
- على سبيل المثال، يمكن لمنصات الاختبار المتعددة المتقدمة اكتشاف مجموعة واسعة من الأجسام المضادة الذاتية في اختبار واحد، مما يوفر ملفات تعريف شاملة للمريض والتي تعد ضرورية لإدارة الحالات المناعية الذاتية المعقدة مثل الذئبة الحمامية الجهازية (SLE) والتهاب المفاصل الروماتويدي.
- تؤدي هذه التطورات إلى تحويل مشهد التشخيص المناعي الذاتي، وتحسين نتائج المرضى، ودفع الطلب على حلول اختبار ANA من الجيل التالي مع حساسية وخصوصية محسنة
ديناميكيات سوق اختبار الأجسام المضادة النووية في أوروبا
سائق
"ارتفاع معدل انتشار أمراض المناعة الذاتية"
- إن الارتفاع المتزايد في حالات الاضطرابات المناعية الذاتية مثل الذئبة الحمامية الجهازية (SLE)، والتهاب المفاصل الروماتويدي، ومتلازمة سجوجرن، والتصلب الجهازي، يدفع بشكل كبير الطلب على اختبارات الأجسام المضادة للنواة
- أصبحت أمراض المناعة الذاتية أكثر شيوعًا بسبب مجموعة من العوامل الوراثية والبيئية ونمط الحياة، مما يزيد من الحاجة إلى التشخيص الدقيق والمبكر لإدارة هذه الحالات المعقدة
- مع تزايد الوعي بشأن اضطرابات المناعة الذاتية، يتبنى مقدمو الرعاية الصحية بشكل متزايد اختبارات ANA كجزء من التشخيص الروتيني لتحسين نتائج المرضى وتقليل تكاليف الرعاية الصحية على المدى الطويل
على سبيل المثال،
- في مارس 2024، وفقًا لتقرير نشرته الجمعية الأوروبية لأمراض الروماتيزم، يُقدر انتشار الذئبة الحمامية الجهازية في أوروبا بنحو 0.1-0.2% من السكان، مع احتياج جزء كبير من هؤلاء المرضى إلى اختبارات ANA بانتظام لإدارة المرض ومراقبته.
- مع استمرار ارتفاع معدل انتشار أمراض المناعة الذاتية، من المتوقع أن ينمو الطلب على اختبارات ANA، مما يدعم التشخيص المبكر والعلاج الشخصي وتحسين نتائج المرضى.
فرصة
"دمج تقنيات التشخيص المتقدمة"
- تقدم التطورات التكنولوجية في اختبار ANA، بما في ذلك الاختبارات المتعددة والمنصات الآلية وأدوات التشخيص التي تعتمد على الذكاء الاصطناعي (AI)، فرص نمو كبيرة في السوق
- تتيح هذه التقنيات تشخيصًا أسرع وأكثر دقة وفعالية من حيث التكلفة من خلال الكشف المتزامن عن العديد من الأجسام المضادة الذاتية، مما يقلل من أوقات الاستجابة ويقلل من الخطأ البشري
- بالإضافة إلى ذلك، يمكن للأنظمة المدعومة بالذكاء الاصطناعي تحليل نتائج الاختبارات في الوقت الفعلي، مما يوفر رؤى تنبؤية حول تطور المرض ومساعدة الأطباء على اتخاذ قرارات أكثر استنارة
على سبيل المثال ،
- في يناير 2025، ووفقًا لدراسة نُشرت في مجلة المناعة الذاتية، أظهرت خوارزميات الذكاء الاصطناعي المُطوّرة لاختبار الأجسام المضادة للنواة (ANA) دقةً أعلى في الكشف عن أمراض المناعة الذاتية في مراحلها المبكرة، مما قلل من النتائج الإيجابية الخاطئة، وحسّن كفاءة التشخيص بشكل عام. يمكن أن يُحسّن هذا التكامل نتائج المرضى بشكل ملحوظ من خلال تسهيل التدخل المبكر والعلاجات المُوجّهة.
- ومن المتوقع أن يؤدي اعتماد هذه التقنيات المتقدمة إلى دفع نمو السوق، حيث تسعى المختبرات ومقدمو الرعاية الصحية إلى تعزيز القدرات التشخيصية ورعاية المرضى.
ضبط النفس/التحدي
"التكلفة العالية لأنظمة التشخيص المتقدمة"
- على الرغم من الفوائد، فإن التكلفة العالية لأنظمة اختبار ANA المتقدمة ومنصات الإرسال المتعددة تشكل عائقًا كبيرًا أمام نمو السوق، وخاصة بالنسبة للمختبرات الصغيرة ومرافق الرعاية الصحية ذات الميزانيات المحدودة.
- يمكن أن يكون الاستثمار الأولي المطلوب للأنظمة الآلية ومعدات الاختبار المتخصصة باهظ التكلفة، مما يؤثر على القدرة على تحمل تكاليف التشخيصات المتقدمة وإمكانية الوصول إليها
- يمكن أن يؤدي هذا العبء المالي إلى تأخير تبني التقنيات المتطورة، وخاصة في المناطق ذات ميزانيات الرعاية الصحية المحدودة، مما يحد من نطاق التشخيصات المناعية الذاتية المتقدمة
على سبيل المثال،
- في نوفمبر 2024، وفقًا لتقرير صادر عن جمعية مصنعي التشخيص الأوروبية، تظل التكلفة العالية لمنصات الاختبار المتعددة وأنظمة اختبار ANA الآلية تشكل تحديًا بالغ الأهمية، حيث تكافح المختبرات الأصغر لتبرير الاستثمار دون نمو كبير في الحجم أو دعم السداد
- وبالتالي، فإن حاجز التكلفة هذا يمكن أن يؤدي إلى تفاوت في جودة التشخيص، مما يحد من الوصول إلى الكشف المبكر والدقيق عن الأمراض المناعية الذاتية للعديد من المرضى.
نطاق سوق اختبار الأجسام المضادة النووية في أوروبا
يتم تقسيم السوق على أساس نوع الجسم المضاد والمنتج والتقنية والتطبيق والمستخدم النهائي وقناة التوزيع.
|
التجزئة |
التجزئة الفرعية |
|
حسب نوع الجسم المضاد |
|
|
حسب المنتج |
|
|
حسب التقنية |
|
|
حسب الطلب |
|
|
حسب المستخدم النهائي |
|
|
حسب قناة التوزيع |
|
في عام 2025، من المتوقع أن تهيمن المناعة الفلورية غير المباشرة (IIF) على السوق بحصة أكبر في قطاع التقنية
من المتوقع أن تهيمن تقنية المناعة الفلورية غير المباشرة (IIF) على سوق اختبارات الأجسام المضادة النووية في أوروبا، مستحوذةً على أكبر حصة بنسبة 59.9% بحلول عام 2025. وتعود هذه الهيمنة بشكل رئيسي إلى مكانتها كمعيار ذهبي لاختبارات الأجسام المضادة للنواة (ANA)، والمعروفة بحساسيتها العالية وقدرتها على اكتشاف مجموعة واسعة من الأجسام المضادة الذاتية. وتُسهم قدرة IIF على تحديد أنماط التلوين المختلفة بشكل كبير في تشخيص العديد من أمراض المناعة الذاتية، مما يجعلها الخيار الأمثل للأطباء. ويُعدّ التطور المستمر في تقنية IIF، إلى جانب الانتشار المتزايد لاضطرابات المناعة الذاتية، من العوامل الرئيسية التي تُعزز ريادتها في السوق.
من المتوقع أن تشكل المستضدات النووية القابلة للاستخراج (ENA) الحصة الأكبر خلال فترة التنبؤ في سوق أنواع الأجسام المضادة
في عام 2025، من المتوقع أن يهيمن قطاع المستضدات النووية القابلة للاستخراج (ENA) على السوق بحصة سوقية تبلغ 34.7%، وذلك بفضل أهميته التشخيصية العالية في أمراض الروماتيزم المناعية الذاتية الجهازية (SARDs)، بما في ذلك الذئبة الحمامية الجهازية، ومتلازمة شوغرن، والتصلب الجهازي. وتعزز قدرة لوحات المستضدات النووية القابلة للاستخراج على اكتشاف العديد من الأجسام المضادة الذاتية في آن واحد دقة وكفاءة التشخيص. كما أن زيادة الوعي بين الأطباء، والتطورات في تقنيات الفحص المتعدد، وتزايد انتشار أمراض المناعة الذاتية، تدعم مكانة هذا القطاع الرائدة في السوق.
تحليل إقليمي لسوق اختبار الأجسام المضادة النووية في أوروبا
- تستحوذ أوروبا الغربية على حصة مهيمنة في سوق اختبارات الأجسام المضادة النووية في أوروبا، بفضل بنيتها التحتية الصحية الراسخة، وتقنياتها الطبية المتقدمة، وطلبها المرتفع على حلول التشخيص المتخصصة. وتستحوذ أوروبا الغربية على ما يقارب 28% من سوق اختبارات الأجسام المضادة النووية في أوروبا.
- ألمانيا هي الدولة الرائدة في أوروبا بحصة سوقية تبلغ 25.6%. وذلك بفضل نظام الرعاية الصحية المتقدم لديها، والوعي العالي لدى المرضى، ووجود تقنيات الاختبار المتقدمة.
- تتمتع المملكة المتحدة بحصة كبيرة في سوق اختبار الأجسام المضادة النووية في أوروبا، ويعزى ذلك إلى البنية التحتية القوية للرعاية الصحية والطلب المتزايد على الاختبارات التشخيصية المتخصصة
- من المتوقع أن تشهد إيطاليا أعلى معدل نمو سنوي مركب (CAGR) بنسبة 13.4% في السوق خلال فترة التوقعات، وذلك نتيجةً لزيادة أمراض المناعة الذاتية، مثل الذئبة، والتهاب المفاصل الروماتويدي، ومتلازمة شوغرن. وقد أدى هذا العبء المرضي المتزايد إلى زيادة الطلب على الاختبارات التشخيصية المبكرة والدقيقة، بما في ذلك اختبارات الأجسام المضادة للنواة (ANA).
- تشهد دول أوروبا الشرقية، بما في ذلك بولندا وروسيا والمجر، نموًا سريعًا في السوق نتيجةً لزيادة استثمارات الرعاية الصحية، وتزايد الوعي، والتحول نحو حلول الرعاية الصحية الحديثة. وتشير التقديرات إلى أن مساهمة أوروبا الشرقية في سوق اختبارات الأجسام المضادة للنواة (ANA) في أوروبا تبلغ حوالي 5-6%.
- يُعزز التوجه المتزايد نحو الرعاية الصحية المنزلية في أوروبا، وخاصةً في دول مثل هولندا والسويد، الطلب على خدمات فحص الأجسام المضادة للنواة (ANA). ويتزايد علاج المرضى في المنزل من الأمراض المزمنة، مما يُعزز اعتماد أدوات التشخيص المتقدمة. وتُمثل حلول التشخيص المتعلقة بالرعاية الصحية المنزلية في أوروبا حوالي 4% من سوق فحص الأجسام المضادة للنواة في أوروبا.
حصة سوق اختبار الأجسام المضادة النووية في أوروبا
يُقدم المشهد التنافسي في السوق تفاصيل لكل منافس. تشمل هذه التفاصيل لمحة عامة عن الشركة، وبياناتها المالية، وإيراداتها المحققة، وإمكانياتها السوقية، والاستثمار في البحث والتطوير، ومبادراتها التسويقية الجديدة، وحضورها العالمي، ومواقع ومرافق الإنتاج، وقدراتها الإنتاجية، ونقاط قوتها وضعفها، وإطلاق المنتجات، ونطاقها، وهيمنة تطبيقاتها. تتعلق نقاط البيانات المذكورة أعلاه فقط بتركيز الشركات على السوق.
الشركات الرائدة الرئيسية العاملة في السوق هي:
- بيونتيك إس إي (ألمانيا)
- جينماب أ/س (الدنمارك)
- شركة إيفوتيك إس إي (ألمانيا)
- شركة جريفولس ش.م. (إسبانيا)
- شركة كريسبر ثيرابيوتكس (سويسرا)
- شركة ثيرمو فيشر العلمية (الولايات المتحدة)
- ترينيتي بيوتك أيرلندا (أيرلندا)
- EUROIMMUN Medizinische Labordiagnostika AG (ألمانيا)
أحدث التطورات في سوق اختبار الأجسام المضادة النووية في أوروبا
- في يونيو 2023، أطلقت شركة EUROIMMUN Medizinische Labordiagnostika AG (ألمانيا)، التابعة لشركة Revvity، جهاز UNIQO 160، وهو نظام متقدم لاختبار المناعة الفلورية غير المباشرة (IIFT) آلي. يُؤتمت هذا النظام عملية IIFT بأكملها، من تحضير العينة إلى تحليل الصور، مما يُعزز كفاءة التشخيص وموثوقيته في اختبارات أمراض المناعة الذاتية.
- في مايو 2023، طرحت شركة Thermo Fisher Scientific مجموعة الفحص المناعي الذاتي، المصممة لتوفير نتائج سريعة ودقيقة للكشف عن أمراض المناعة الذاتية. يهدف هذا المنتج الجديد إلى تحسين قدرات التشخيص وإدارة المرضى.
- في مارس 2023، أطلقت شركة ترينيتي بيوتيك جهاز Autoimmune Panel Plus، وهو اختبار تشخيصي يوفر دقة وسرعة أكبر في الكشف عن مختلف أمراض المناعة الذاتية. يُحسّن هذا المنتج بشكل كبير سير العمل التشخيصي في المختبرات السريرية.
- في يونيو 2022، أبرمت شركة ثيرادياج شراكة مع شركة كوتينت المحدودة لتطوير تشخيصات المناعة الذاتية بالاستفادة من منصة موزايك التابعة لشركة كوتينت. وبموجب هذه الاتفاقية، تزود ثيرادياج شركة كوتينت بكواشف المناعة الذاتية وأنظمة مراقبة الجودة لتطوير مصفوفات المناعة الذاتية الدقيقة، مع التركيز في أول تطبيق على أمراض النسيج الضام (CTD).
- في مايو 2022، حصلت شركة ZEUS Scientific على موافقة إدارة الغذاء والدواء الأمريكية (FDA) على نظامها المناعي الرقمي dIFine، والذي يُستخدم مع اختبار الأجسام المضادة الفلورية غير المباشرة (IFA) لـ ANA HEp-2 من ZEUS. يتضمن هذا الترخيص تحديدًا إيجابيًا وسلبيًا وثمانية أنماط شائعة لتلوين ANA HEp-2، مما يُسهم في تحسين دقة التشخيص.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.