Asia Pacific Multiple Hereditary Exostosis Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
22.90 Million
USD
33.33 Million
2024
2032
USD
22.90 Million
USD
33.33 Million
2024
2032
| 2025 –2032 | |
| USD 22.90 Million | |
| USD 33.33 Million | |
|
|
|
|
تجزئة سوق النتوءات العظمية الوراثية المتعددة في منطقة آسيا والمحيط الهادئ، حسب النوع (الجلوس والمنثني)، العلاج (الجراحة، الأدوية، وغيرها)، التشخيص (الأشعة السينية، التصوير المقطعي المحوسب (CT)، التصوير بالرنين المغناطيسي (MRI)، الاختبارات الجينية، وغيرها)، الموقع (الساقين، الذراعين، الكتفين، الحوض، أصابع اليدين والقدمين)، الفئة العمرية (الأطفال والبالغين)، المستخدم النهائي (المستشفيات، العيادات التخصصية، مراكز الجراحة الخارجية، وغيرها) - اتجاهات الصناعة والتوقعات حتى عام 2032
حجم سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
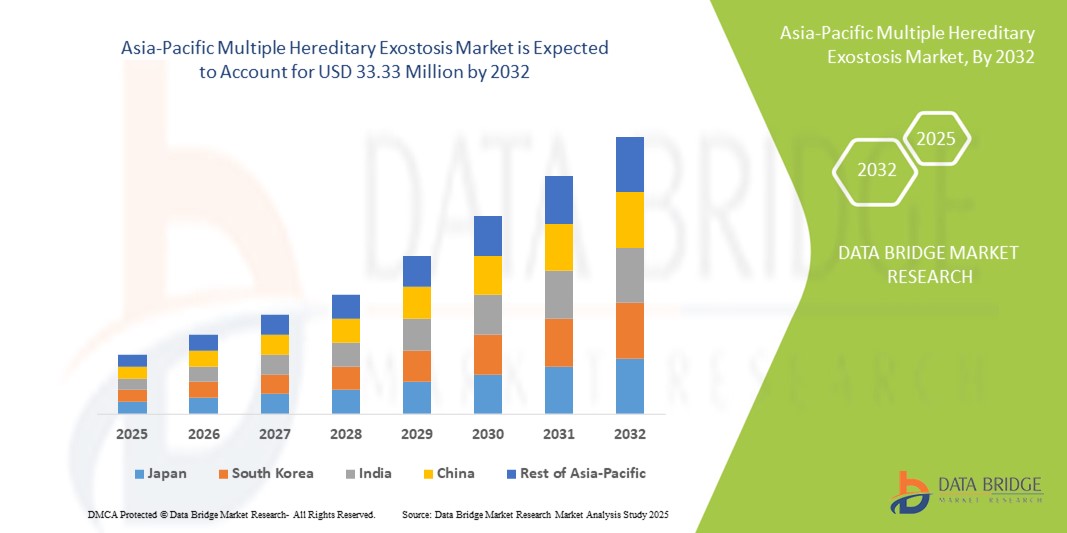
- تم تقييم حجم سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ بـ 22.90 مليون دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 33.33 مليون دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 4.80٪ خلال الفترة المتوقعة
- يعود نمو السوق إلى حد كبير إلى زيادة الوعي والتشخيص المبكر للاضطرابات الوراثية النادرة، وتحسين الوصول إلى الرعاية الصحية المتخصصة، وزيادة برامج الفحص الجيني في الاقتصادات الناشئة في المنطقة.
- علاوة على ذلك، فإن تزايد الاستثمارات في الطب الدقيق، والمبادرات الحكومية الداعمة، وتزايد أعداد مرضى الأطفال، كلها عوامل تُعزز الطلب على خيارات العلاج المتقدمة. تُسرّع هذه العوامل المتقاربة من اعتماد الحلول التشخيصية والعلاجية، مما يُعزز نمو هذا القطاع بشكل كبير.
تحليل سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
- إن الخلل الوراثي المتعدد (MHE)، وهو اضطراب وراثي نادر يتميز بأورام حميدة في العظام، يحظى باهتمام سريري متزايد في منطقة آسيا والمحيط الهادئ بسبب زيادة الوعي وتحسين القدرات التشخيصية وتزايد إمكانية الوصول إلى خدمات العظام والوراثة المتخصصة في المراكز الحضرية.
- إن الطلب المتزايد على التشخيص المبكر والتدخلات العلاجية المتقدمة مدفوع في المقام الأول بالبنية التحتية المحسنة للرعاية الصحية، والدعم الحكومي لإدارة الأمراض النادرة، وتوسيع برامج الفحص الجيني في دول مثل الصين واليابان والهند.
- سيطرت الصين على سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ بأكبر حصة إيرادات بلغت 39.9% في عام 2024، بدعم من تزايد عدد مرضى الأطفال، والتقدم في الطب الجينومي، ووجود مراكز إقليمية للتميز تركز على خلل التنسج الهيكلي.
- من المتوقع أن تكون الهند أسرع دولة نموًا في سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ خلال فترة التوقعات، مدفوعة بسياسات الأمراض النادرة المحسنة، وتوسيع نشاط التجارب السريرية، والدور المتزايد لمنظمات الدفاع عن المرضى.
- سيطرت شريحة العظام الثابتة على سوق النخر العظمي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ بحصة سوقية بلغت 59.1% في عام 2024، مدفوعًا بانتشارها المرتفع بالقرب من المفاصل وزيادة احتمالية التسبب في ضغط الأعصاب، مما دفع إلى الكشف السريري المبكر والتدخل.
نطاق التقرير وتجزئة سوق النخر العظمي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ
|
صفات |
رؤى رئيسية حول سوق التكاثر الخارجي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
آسيا والمحيط الهادئ
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تشمل أيضًا تحليلًا متعمقًا من الخبراء، وتحليل التسعير، وتحليل حصة العلامة التجارية، واستطلاع رأي المستهلكين، وتحليل التركيبة السكانية، وتحليل سلسلة التوريد، وتحليل سلسلة القيمة، ونظرة عامة على المواد الخام / المواد الاستهلاكية، ومعايير اختيار البائعين، وتحليل PESTLE، وتحليل Porter، والإطار التنظيمي. |
اتجاهات سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
"التطورات في التشخيص الجيني وأساليب العلاج الشخصية"
- من الاتجاهات المهمة والمتسارعة في سوق التشوهات الخلقية الوراثية المتعددة (MHE) في منطقة آسيا والمحيط الهادئ التكامل المتزايد لأدوات التشخيص الجيني المتقدمة وظهور بروتوكولات علاجية شخصية مصممة خصيصًا لتلبية الاحتياجات المحددة لمرضى الأطفال المصابين بخلل التنسج الهيكلي. يُحسّن هذا الاتجاه الكشف المبكر ويُمكّن من وضع استراتيجيات تدخل مُستهدفة.
- على سبيل المثال، تُستخدم تقنيات تسلسل الجيل التالي (NGS) بشكل متزايد في دول مثل اليابان والصين لتحديد طفرات جينات EXT1 وEXT2، مما يسمح للأطباء بتشخيص اعتلال الدماغ الخلقي الخلقي (MHE) مبكرًا وبدقة. كما أصبحت الاستشارة الوراثية ممارسة شائعة في عيادات العظام وطب الأطفال المتخصصة.
- يُتيح الاستخدام المتزايد لتقنيات التصوير غير الجراحي، مثل التصوير المقطعي المحوسب منخفض الإشعاع والتصوير بالرنين المغناطيسي عالي الدقة، مراقبةً أفضل لتطور النتوءات العظمية، ويساعد في التخطيط الجراحي. إلى جانب التحليل الإشعاعي المُعزز بالذكاء الاصطناعي، تُوفر هذه الأدوات تتبعًا أكثر دقة للآفات وتخصيصًا للعلاج.
- إن هذه التطورات التشخيصية، إلى جانب حملات التوعية الصحية العامة وبرامج الفحص في المراكز الحضرية، تعمل على تعزيز التحول نحو الإدارة الاستباقية للمرض بدلاً من التدخل الجراحي التفاعلي فقط بعد ظهور المضاعفات.
- تتزايد سجلات المرضى والتعاون عبر الحدود في جميع أنحاء المنطقة، حيث تشارك دول مثل كوريا الجنوبية وأستراليا في شبكات الأمراض النادرة التي تُسهّل البحث السريري وتبادل البيانات حول الصحة العقلية. تُساعد هذه المبادرات على توليد رؤى وبائية خاصة بكل منطقة، وتعزيز الرعاية القائمة على الأدلة.
- يؤدي هذا التركيز المتزايد على التعرف المبكر والرعاية الشخصية إلى تحويل مشهد علاج MHE في منطقة آسيا والمحيط الهادئ، مما يخلق منصة للابتكار الطبي وتقديم حلول تقويم العظام التي تركز على المريض.
ديناميكيات سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
سائق
"ارتفاع معدلات التشخيص المبكر وتوسع خدمات طب العظام للأطفال"
- يُعدّ تزايد توافر الرعاية المتخصصة في جراحة العظام للأطفال والبنية التحتية التشخيصية في اقتصادات آسيا والمحيط الهادئ الرئيسية دافعًا رئيسيًا لنمو سوق الرعاية الصحية للأطفال. وقد أتاح الكشف المبكر من خلال الفحص الجيني وتحسين الوصول إلى التصوير الطبي المتقدم إجراء تدخلات مبكرة وتحسين نتائج المرض.
- على سبيل المثال، أدت المبادرات الحكومية في الهند والصين لإدراج الأمراض النادرة في برامج الصحة الوطنية إلى دمج التعليم الصحي الأساسي في لجان التشخيص بالمستشفيات الجامعية، مما أدى إلى ارتفاع معدلات تشخيص الحالات. كما يجري تطوير سجلات وطنية للأمراض النادرة لرصد النتائج على المدى الطويل.
- مع ازدياد الوعي بين الأطباء والعائلات، ازداد الطلب على خدمات التصحيح الجراحي للتشوهات وإدارة الألم، لا سيما في المستشفيات المتخصصة بطب الأطفال ومراكز جراحة العظام. ويتزايد استخدام منصات الجراحة بمساعدة الروبوت والطباعة ثلاثية الأبعاد لنمذجة العظام لاستئصال النتوءات العظمية بدقة.
- كما أن ظهور منظمات دعم الأمراض النادرة في المنطقة يُساعد المرضى وعائلاتهم على الوصول إلى الموارد، ويدفع باتجاه بروتوكولات رعاية أفضل، ويربطهم بمقدمي الرعاية المتخصصين. تُعزز هذه الجهود المشاركة المبكرة والعلاج الشامل لهذه الحالة.
ضبط النفس/التحدي
"انخفاض الوعي في المناطق الريفية ومحدودية الوصول إلى العلاج المتخصص"
- على الرغم من التقدم المحرز في مراكز الرعاية الصحية الحضرية، إلا أن محدودية الوعي باضطرابات الصحة الوراثية لدى الأطباء العامين، وقلة توافر مرافق التشخيص الجيني في المناطق الريفية أو النائية، لا تزال تُشكل عائقًا رئيسيًا أمام انتشارها على نطاق واسع. لا تُشخص العديد من الحالات أو تُشخص خطأً بسبب ندرة الحالة وتعقيد أعراضها.
- في البلدان ذات البنية التحتية للرعاية الصحية الأقل قوة، مثل أجزاء من جنوب شرق آسيا، قد يواجه المرضى تأخيرات في الوصول إلى أخصائيي العظام أو قد يفتقرون إلى الوسائل المالية لإجراء الاختبارات الجينية والتصوير المتابعة، مما يؤدي إلى نقص العلاج أو حدوث مضاعفات يمكن تجنبها.
- علاوة على ذلك، يُصعّب غياب إرشادات علاجية موحدة وأنظمة إحالة مجزأة على الأفراد المصابين الحصول على رعاية متعددة التخصصات. وبينما تُنفّذ مبادرات لتحسين أطر سياسات الأمراض النادرة في العديد من دول آسيا والمحيط الهادئ، لا يزال التنفيذ متفاوتًا.
- للتغلب على هذه التحديات، تُعد زيادة الاستثمار في الرعاية الصحية الريفية، ومنصات التطبيب عن بُعد لاستشارات جراحة العظام، وبرامج الفحص الجيني المدعومة، وتدريب الأطباء على الأمراض النادرة، أمورًا أساسية. وستكون الشراكات بين الحكومات والمنظمات غير الحكومية والمؤسسات الطبية حيوية لسد فجوة الوصول وضمان رعاية منصفة لمرضى نقص المناعة البشرية في جميع أنحاء المنطقة.
نطاق سوق النخر العظمي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ
يتم تقسيم السوق على أساس النوع والعلاج والتشخيص والموقع والفئة العمرية والمستخدم النهائي
- حسب النوع
بناءً على النوع، يُقسّم سوق النتوءات العظمية الوراثية المتعددة في منطقة آسيا والمحيط الهادئ إلى نوعين: النتوءات العظمية اللاصقة والناتئة. وقد هيمنت النتوءات العظمية اللاصقة على السوق محققةً أكبر حصة إيرادات بلغت 59.1% في عام 2024، بفضل وضوحها السريري العالي بفضل قاعدتها الواسعة وقربها من الهياكل الحيوية كالأعصاب والمفاصل. وغالبًا ما تؤدي هذه السمات إلى ظهور أعراض مبكرة، وارتفاع معدلات التشخيص، وزيادة الحاجة إلى التدخل الجراحي. كما أن الآفات اللاصقة ترتبط في كثير من الأحيان بضعف وظيفي وتشوهات، مما يستدعي تقييمًا تقويميًا سريعًا.
من المتوقع أن ينمو قطاع الأورام المعنقة بأسرع وتيرة حتى عام ٢٠٣٢، مدعومًا بتحسين قدرات التصوير التي تتيح اكتشافًا أفضل للنتوءات الخارجية طويلة الساق، وتزايد استخدام تقنيات المراقبة غير الجراحية. ومع تزايد الوعي وتوافر أدوات التصوير الإشعاعي في العيادات الخارجية، من المتوقع أيضًا ارتفاع معدلات اكتشاف الآفات المعنقة الأقل أعراضًا.
- حسب العلاج
بناءً على العلاج، يُقسّم سوق النتوءات العظمية الوراثية المتعددة في منطقة آسيا والمحيط الهادئ إلى جراحات وأدوية وغيرها. وقد استحوذ قطاع الجراحة على أكبر حصة سوقية بنسبة 46.7% في عام 2024، نظرًا لدوره المحوري في إدارة المضاعفات مثل انضغاط الأعصاب، وتشوهات الأطراف، أو تقييد حركة المفاصل. غالبًا ما يخضع مرضى الأطفال لاستئصال جراحي للنتوءات العظمية للوقاية من تشوهات النمو أو الألم المزمن. ويدعم نمو هذا القطاع توافر جراحي العظام المهرة والأدوات المتطورة، مثل الجراحة بمساعدة الروبوت والنمذجة ثلاثية الأبعاد، في دول مثل اليابان والصين وكوريا الجنوبية.
من المتوقع أن يشهد قطاع الأدوية أسرع معدل نمو سنوي مركب بين عامي 2025 و2032، ويعود ذلك بشكل رئيسي إلى دعم إدارة الألم بعد الجراحة والسيطرة على الالتهابات. كما يتزايد الاهتمام السريري بالعلاجات الموجهة التي تهدف إلى الحد من نمو النتوءات العظمية الخارجية، مع أن هذه الخيارات لا تزال في معظمها في مرحلة البحث.
- حسب التشخيص
بناءً على التشخيص، يُقسّم سوق النخر العظمي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ إلى الأشعة السينية، والتصوير المقطعي المحوسب (CT)، والتصوير بالرنين المغناطيسي (MRI)، والاختبارات الجينية، وغيرها. هيمنت الأشعة السينية على قطاع التشخيص بنسبة 34.2% في عام 2024، بفضل فعاليتها من حيث التكلفة، وتوافرها الواسع، وكفاءتها في تحديد النمو العظمي المميز للنخر العظمي الوراثي المتعدد. ولا تزال تُعدّ أداة التصوير الأولية في معظم البيئات السريرية.
من المتوقع أن يشهد الفحص الجيني نموًا سريعًا حتى عام ٢٠٣٢ نظرًا لدوره في تأكيد الأنماط الوراثية من خلال تحليل جينات EXT1 وEXT2. ومع تزايد الوعي وإمكانية الوصول إلى تسلسل الجيل التالي (NGS)، لا سيما في المراكز الطبية الحضرية، أصبح الفحص الجيني ضروريًا للتشخيص المبكر والاستشارات الأسرية.
- حسب الموقع
بناءً على موقع الإصابة، يُقسّم سوق النتوءات العظمية الوراثية المتعددة في منطقة آسيا والمحيط الهادئ إلى عظام الساقين، والذراعين، والكتفين، والحوض، وأصابع اليدين، وأصابع القدم. وقد استحوذت عظام الساقين على أعلى حصة بنسبة 36.9% في عام 2024، نظرًا لشيوع حدوث النتوءات العظمية بالقرب من عظام الفخذ، والظنبوب، والشظية، وهي مناطق معرضة للإجهاد الميكانيكي واضطرابات النمو لدى الأطفال. وغالبًا ما تتطلب هذه الإصابات تقييمًا عظاميًا وجراحة تصحيحية بسبب تشوهات المشي أو اختلافات طول الأطراف.
ومن المتوقع أن تشهد الذراعين والكتفين أسرع نمو خلال فترة التوقعات، باعتبارها مواقع تشريحية شائعة، وخاصة في فئات الأطفال، حيث يمكن أن تتداخل التشوهات مع الوظيفة الطبيعية ونطاق الحركة.
- حسب الفئة العمرية
بناءً على الفئة العمرية، يُقسّم سوق النخر العظمي الوراثي المتعدد في منطقة آسيا والمحيط الهادئ إلى قسمين: قسم الأطفال وقسم البالغين. وسيستحوذ قسم الأطفال على حصة سوقية تبلغ 72.1% في عام 2024، حيث يُشخّص النخر العظمي المتعدد عادةً في مرحلة الطفولة المبكرة، عندما يكون نمو العظام نشطًا، وتبدأ أعراض مثل النتوءات المرئية، أو تقييد المفاصل، أو تشوهات الأطراف بالظهور.
وفي حين من المتوقع أن تشهد حالات البالغين أسرع معدل نمو سنوي مركب من عام 2025 إلى عام 2032، وخاصة بسبب التشخيص المتأخر أو تكرار المرض، فإن غالبية التدخلات والفحوصات والعمليات الجراحية تحدث أثناء الطفولة والمراهقة.
- حسب المستخدم النهائي
بناءً على المستخدم النهائي، يُقسّم سوق التشوهات الخلقية الوراثية المتعددة في منطقة آسيا والمحيط الهادئ إلى مستشفيات، وعيادات متخصصة، ومراكز جراحية متنقلة، وغيرها. وقد تصدرت المستشفيات هذا القطاع بحصة بلغت 48.3% في عام 2024، بفضل قدراتها التشخيصية والجراحية الشاملة، بما في ذلك جراحة العظام، والأشعة، والاستشارات الوراثية. وتُعدّ مستشفيات الأطفال والمراكز الطبية التابعة للجامعات في دول مثل الصين واليابان والهند مراكز رئيسية لتشخيص وعلاج التشوهات الخلقية الوراثية المتعددة.
ومن المتوقع أن تشهد مراكز الجراحة الخارجية نموًا قويًا خلال فترة التوقعات، بسبب التفضيل المتزايد للإجراءات الجراحية الأقل تدخلاً وجراحات الرعاية النهارية.
تحليل إقليمي لسوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
- سيطرت الصين على سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ بأكبر حصة إيرادات بلغت 39.9% في عام 2024، بدعم من تزايد عدد مرضى الأطفال، والتقدم في الطب الجينومي، ووجود مراكز إقليمية للتميز تركز على خلل التنسج الهيكلي.
- يتبنى المرضى والأطباء في الصين ودول أخرى مثل اليابان والهند بشكل نشط استراتيجيات التدخل المبكر والتقنيات الجراحية المتقدمة وخطط الرعاية الشخصية، مما يؤدي إلى نتائج أفضل للمرضى وتوسع السوق.
- يتم تعزيز هذا النمو من خلال السياسات الوطنية للأمراض النادرة، والشراكات بين القطاعين العام والخاص في مجال الرعاية الصحية، والتعاون البحثي المستمر، مما يجعل منطقة آسيا والمحيط الهادئ منطقة رائدة في الابتكار والتقدم السريري في تشخيص وعلاج الأمراض النادرة.
نظرة على سوق الأورام الخبيثة الوراثية المتعددة في الصين
استحوذ سوق التشوهات العظمية الوراثية المتعددة في الصين على أكبر حصة إيرادات بنسبة 39.9% في سوق معدات طب العظام الطبية في منطقة آسيا والمحيط الهادئ في عام 2024، مدعومًا بارتفاع عدد الأطفال في هذا السوق، والاعتماد المتزايد على تقنيات التصوير المتقدمة، وتوسيع نطاق الوصول إلى الاختبارات الجينية في المستشفيات الحضرية. تدعم الاستراتيجيات الوطنية للأمراض النادرة، إلى جانب زيادة الاستثمار في الطب الجينومي، التشخيص المبكر وإدارة اضطرابات الهيكل العظمي الوراثية. ويواصل وجود برامج الفحص المدعومة حكوميًا ومراكز العظام المتخصصة دفع نمو السوق.
نظرة عامة على سوق الأورام الخبيثة الوراثية المتعددة في اليابان
يشهد سوق التشوهات الخلقية الوراثية المتعددة في اليابان نموًا ملحوظًا، مدعومًا بالبنية التحتية المتطورة للرعاية الصحية في البلاد والتركيز على الطب الدقيق. يُمكّن دمج الاختبارات الجينية في مسارات التشخيص القياسية، إلى جانب إطار السياسة الاستباقية للأمراض النادرة في اليابان، من الكشف المبكر والدقيق عن التشوهات الخلقية الوراثية المتعددة. كما يُسهم التعاون الوثيق بين معاهد البحث ومقدمي الرعاية الصحية في تحسين بروتوكولات العلاج ونتائج المرضى.
نظرة عامة على سوق النتوءات الخارجية الوراثية المتعددة في الهند
سيستحوذ سوق النتوءات العظمية الوراثية المتعددة في الهند على حصة كبيرة من سوق الرعاية الصحية الأولية في منطقة آسيا والمحيط الهادئ بحلول عام 2024، مدفوعًا بتزايد توافر أخصائيي العظام، وتحسين أدوات التشخيص في المستشفيات الجامعية، وازدياد الوعي العام. تُمكّن المبادرات الحكومية الرامية إلى تحديد الأمراض النادرة، بما في ذلك السياسة الوطنية للأمراض النادرة، من تحسين الوصول إلى التشخيص المبكر والعلاج الجراحي. كما أن وجود شريحة كبيرة من الأطفال وتوسع البنية التحتية للرعاية الصحية في المراكز الحضرية يدعم نمو السوق.
حصة سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ
تعد صناعة الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ بقيادة شركات راسخة بشكل أساسي، بما في ذلك:
- شركة إيلومينا (الولايات المتحدة)
- ميدترونيك (أيرلندا)
- شركة زيمر بيوميت القابضة المحدودة (الولايات المتحدة)
- سترايكر (الولايات المتحدة)
- شركة سيمنز هيلثينيرز إيه جي (ألمانيا)
- شركة GE HealthCare Technologies Inc. (الولايات المتحدة)
- شركة كانون للأنظمة الطبية (اليابان)
- إيساوتي سبا (إيطاليا)
- شركة ثيرمو فيشر العلمية (الولايات المتحدة)
- شركة روش القابضة (سويسرا)
- كونينكليكي فيليبس إن في (هولندا)
- B. Braun Melsungen AG (ألمانيا)
- شركة أجيلنت تكنولوجيز (الولايات المتحدة)
- شركة بيركين إلمر (الولايات المتحدة)
- شركة فوجي فيلم (اليابان)
- معهد بكين لعلم الجينوم (BGI Genomics) (الصين)
- شركة شنتشن ميندراي للإلكترونيات الطبية الحيوية المحدودة (الصين)
- شركة جيانغسو تراوتيك للتكنولوجيا الطبية المحدودة (الصين)
- شركة توشيبا (اليابان)
- شركة تاكارا بيو (اليابان)
ما هي التطورات الأخيرة في سوق الأورام الخبيثة الوراثية المتعددة في منطقة آسيا والمحيط الهادئ؟
- في مايو 2024، وسّعت وزارة الصحة الصينية سجلها الوطني للأمراض النادرة ليشمل النتوءات الوراثية المتعددة (MHE)، بهدف تحسين التشخيص المبكر، وتوفير العلاج، والمراقبة طويلة الأمد للمرضى في جميع المقاطعات الرئيسية. تعكس هذه المبادرة الاستثمار الاستراتيجي للصين في إدارة الأمراض النادرة، والتزامها باستخدام أنظمة البيانات المركزية لرعاية المرضى الشخصية وتحسين النتائج السريرية.
- في مارس 2024، أطلق المركز الوطني الياباني لصحة الطفل ونموه مشروعًا بحثيًا تعاونيًا يركز على علاجات تعديل الجينات التي تستهدف طفرات EXT1 وEXT2 في حالات اعتلال الدماغ الهيكلي النقوي (MHE). وتشارك في هذه المبادرة جامعات رائدة وشركات تكنولوجيا حيوية، مما يمثل تقدمًا ملحوظًا في الطب الدقيق وابتكار العلاج الجيني لاضطرابات الهيكل العظمي النادرة في البلاد.
- في فبراير 2024، وافق المجلس الهندي للبحوث الطبية (ICMR) على تمويل برنامج تجريبي وطني يركز على الفحص الجيني للمرضى الأطفال المشتبه بإصابتهم بخلل التنسج الهيكلي، بما في ذلك خلل التنسج الهيكلي الخلقي. صُمم البرنامج لتعزيز التشخيص المبكر، وبناء القدرات التشخيصية في المستشفيات العامة، ودعم الأسر من خلال خدمات الاستشارة الوراثية.
- في يناير 2024، بدأ مستشفى جامعة سيول الوطنية في كوريا الجنوبية تجارب سريرية على خوارزميات التصوير بمساعدة الذكاء الاصطناعي لتحسين دقة تشخيص العاهات العظمية الصغرى من خلال تفسير فحوصات الرنين المغناطيسي والتصوير المقطعي المحوسب. يُبرز هذا التطور سعي كوريا الجنوبية نحو دمج الذكاء الاصطناعي في تشخيصات العظام، مما يُعزز الكفاءة ويُقلل من تأخير التشخيص.
- في ديسمبر 2023، أعلنت الشبكة الأسترالية للأمراض النادرة (ARDN) عن شراكة مع العديد من مستشفيات الأطفال لتوحيد بروتوكولات علاج اعتلال عظام الأطفال الخلقي (MHE) واضطرابات العظام الأخرى. يشمل التعاون وحدات تدريبية لمقدمي الرعاية الصحية، وتطوير سجل رقمي مركزي للمرضى لتسهيل البحث واتخاذ القرارات السريرية وصياغة السياسات.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE ANALYSIS - OBSERVATORY DATA
4.4 EPIDEMIOLOGY
5 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: REGULATIONS
5.1 REGULATION IN UNITED STATES: U.S. FOOD AND DRUG ADMINISTRATION (FDA)
5.1.1 REGULATION IN EUROPE: EUROPEAN MEDICINES AGENCY (EMA)
5.2 REGULATION IN ASIA-PACIFIC (JAPAN): PHARMACEUTICAL AND MEDICAL DEVICES AGENCY (PMDA)
5.3 MEDICAL DEVICE STANDARDS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF GENETIC DISORDERS
6.1.2 GROWING PEDIATRIC POPULATION
6.1.3 DEVELOPMENT OF NOVEL THERAPIES
6.2 RESTRAINTS
6.2.1 HIGH COST OF ADVANCED THERAPIES
6.2.2 LIMITED AVAILABILITY OF THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASE IN PATIENT CARE AND SUPPORT SYSTEMS
6.3.2 INCREASE IN THE NUMBER OF COLLABORATIONS AND PARTNERSHIPS
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS ABOUT THE DISORDER
6.4.2 LACK OF DRUG APPROVALS ASSOCIATED WITH THE DISORDER
7 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE
7.1 OVERVIEW
7.2 SESSILE
7.3 PEDUNCULATED
8 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT
8.1 OVERVIEW
8.2 SURGERY
8.2.1 REMOVE THE TUMOR
8.2.2 LENGTHEN LIMBS
8.3 MEDICATION
8.3.1 HOSPITAL PHARMACIES
8.3.2 DRUGS STORES AND RETAIL PHARMACIES
8.3.3 ONLINE PHARMACIES
8.4 OTHERS
9 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS
9.1 OVERVIEW
9.2 X-RAY
9.2.1 SESSILE
9.2.2 PEDUNCULATED
9.3 COMPUTED TOMOGRAPHY (CT) SCAN
9.3.1 SESSILE
9.3.2 PEDUNCULATED
9.4 MAGNETIC RESONANCE IMAGING (MRI)
9.4.1 SESSILE
9.4.2 PEDUNCULATED
9.5 GENETIC TESTS
9.5.1 SESSILE
9.5.2 PEDUNCULATED
9.6 OTHERS
9.6.1 SESSILE
9.6.2 PEDUNCULATED
10 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE
10.1 OVERVIEW
10.2 LEGS
10.3 ARMS
10.4 SHOULDERS
10.5 PELVIS
10.6 FINGERS
10.7 TOES
11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
12 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.2.1 PRIVATE
12.2.2 GOVERNMENT
12.3 SPECIALTY CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 INDIA
13.1.4 AUSTRALIA
13.1.5 SOUTH KOREA
13.1.6 INDONESIA
13.1.7 NEW ZEALAND
13.1.8 MALAYSIA
13.1.9 THAILAND
13.1.10 TAIWAN
13.1.11 SINGAPORE
13.1.12 PHILIPPINES
13.1.13 VIETNAM
13.1.14 REST OF ASIA-PACIFIC
14 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 BAYERS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT UPDATES
16.2 HALEON GROUP OF COMPANIES
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 BASF
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT UPDATES
16.4 VIATRIS INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT UPDATES
16.5 ACTIZAPHARMA
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT UPDATES
16.6 ADVACARE PHARMA
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT UPDATES
16.7 AUROBINDO PHARMA
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT TABLETS
16.8 HALEON GROUP OF COMPANIES
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT UPDATES
16.9 IPSEN BIOPHARMACEUTICALS, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT UPDATES
16.1 MALLINCKRODT
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT UPDATES
16.11 TEVA PHARMACEUTICAL INDUSTRIES LTD.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT UPDATES
16.12 TAJ PHARMACEUTICALS LIMITED
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 PIPELINE ANALYSIS - OBSERVATORY DATA
TABLE 2 PIPELINE ANALYSIS - INTERVENTIONAL DATA
TABLE 3 SALES DATA - 2024
TABLE 4 SALES DATA - 2023
TABLE 5 SALES DATA - 2022
TABLE 6 SALES DATA - 2021
TABLE 7 SALES DATA - 2020
TABLE 8 SALES DATA - 2019
TABLE 9 SALES DATA - 2018
TABLE 10 COST OF PALOVAROTENE
TABLE 11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 12 ASIA-PACIFIC SESSILE IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 13 ASIA-PACIFIC PEDUNCULATED IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 14 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 15 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 16 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 18 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 19 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 20 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 21 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 22 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 23 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 24 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 25 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 26 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 27 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 28 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 29 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 32 ASIA-PACIFIC LEGS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 ASIA-PACIFIC ARMS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 ASIA-PACIFIC SHOULDERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 ASIA-PACIFIC PELVIS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 36 ASIA-PACIFIC FINGERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 ASIA-PACIFIC TOES IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 38 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 39 ASIA-PACIFIC PEDIATRIC IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 40 ASIA-PACIFIC ADULT IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 41 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 42 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 44 ASIA-PACIFIC SPECIALTY CLINICS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 ASIA-PACIFIC AMBULATORY SURGICAL CENTERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 47 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 48 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 49 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 50 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 59 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 60 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 61 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 63 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 64 CHINA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 CHINA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 66 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 67 CHINA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 68 CHINA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 CHINA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 CHINA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 71 CHINA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 72 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 73 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 74 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 75 CHINA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 78 JAPAN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 JAPAN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 80 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 81 JAPAN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 JAPAN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 83 JAPAN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 JAPAN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 JAPAN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 87 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 88 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 89 JAPAN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 91 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 92 INDIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 INDIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 94 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 95 INDIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 INDIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 INDIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 INDIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 INDIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 101 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 102 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 103 INDIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 104 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 105 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 106 AUSTRALIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 107 AUSTRALIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 108 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 109 AUSTRALIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 110 AUSTRALIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 111 AUSTRALIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 112 AUSTRALIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 113 AUSTRALIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 114 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 115 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 116 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 117 AUSTRALIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 118 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 119 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 120 SOUTH KOREA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 121 SOUTH KOREA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 122 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 123 SOUTH KOREA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 124 SOUTH KOREA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 125 SOUTH KOREA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 126 SOUTH KOREA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 127 SOUTH KOREA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 128 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 129 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 130 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 131 SOUTH KOREA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 132 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 133 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 134 INDONESIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 135 INDONESIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 136 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 137 INDONESIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 138 INDONESIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 139 INDONESIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 140 INDONESIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 141 INDONESIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 142 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 143 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 144 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 145 INDONESIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 146 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 147 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 148 NEW ZEALAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 149 NEW ZEALAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 150 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 151 NEW ZEALAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 152 NEW ZEALAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 153 NEW ZEALAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 154 NEW ZEALAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 155 NEW ZEALAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 156 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 157 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 158 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 159 NEW ZEALAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 160 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 161 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 162 MALAYSIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 163 MALAYSIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 164 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 165 MALAYSIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 166 MALAYSIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 167 MALAYSIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 168 MALAYSIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 169 MALAYSIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 170 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 171 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 172 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 173 MALAYSIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 174 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 175 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 176 THAILAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 177 THAILAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 178 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 179 THAILAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 180 THAILAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 181 THAILAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 182 THAILAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 183 THAILAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 184 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 185 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 186 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 187 THAILAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 188 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 189 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 190 TAIWAN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 191 TAIWAN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 192 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 193 TAIWAN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 194 TAIWAN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 195 TAIWAN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 196 TAIWAN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 197 TAIWAN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 198 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 199 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 200 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 201 TAIWAN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 202 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 203 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 204 SINGAPORE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 205 SINGAPORE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 206 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 207 SINGAPORE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 208 SINGAPORE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 209 SINGAPORE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 210 SINGAPORE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 211 SINGAPORE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 212 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 213 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 214 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 215 SINGAPORE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 216 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 217 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 218 PHILIPPINES SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 219 PHILIPPINES MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 220 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 221 PHILIPPINES X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 222 PHILIPPINES COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 223 PHILIPPINES MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 224 PHILIPPINES GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 225 PHILIPPINES OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 226 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 227 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 228 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 229 PHILIPPINES HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 230 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 231 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 232 VIETNAM SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 233 VIETNAM MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 234 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 235 VIETNAM X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 236 VIETNAM COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 237 VIETNAM MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 238 VIETNAM GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 239 VIETNAM OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 240 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 241 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 242 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 243 VIETNAM HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 244 REST OF ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
List of Figure
FIGURE 1 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: ASIA-PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 PRODUCT LIFELINE CURVE
FIGURE 9 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 TWO SEGMENTS COMPRISE THE ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET, BY TYPE
FIGURE 15 RISING PREVALENCE OF GENETIC DISORDERS IS DRIVING THE GROWTH OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET FROM 2024 TO 2031
FIGURE 16 THE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET IN 2024 AND 2031
FIGURE 17 MARKET OVERVIEW
FIGURE 18 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2023
FIGURE 19 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2023
FIGURE 23 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2024-2031 (USD THOUSAND)
FIGURE 24 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 26 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2023
FIGURE 27 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2024-2031 (USD THOUSAND)
FIGURE 28 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, CAGR (2024-2031)
FIGURE 29 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, LIFELINE CURVE
FIGURE 30 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2023
FIGURE 31 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2024-2031 (USD THOUSAND)
FIGURE 32 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, CAGR (2024-2031)
FIGURE 33 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, LIFELINE CURVE
FIGURE 34 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2023
FIGURE 35 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2024-2031 (USD THOUSAND)
FIGURE 36 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, CAGR (2024-2031)
FIGURE 37 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 38 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2023
FIGURE 39 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 40 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, CAGR (2024-2031)
FIGURE 41 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET: SNAPSHOT (2023)
FIGURE 43 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY SHARE 2023 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.