إن المشاركة المتكررة للمنظمات الصحية الحكومية تضمن أعلى مستوى من سلامة المرضى. أنشأت إدارة الغذاء والدواء الأمريكية (FDA) مكتب المنتجات المركبة (OCP) لمعالجة المشكلات المرتبطة بالمنتجات المركبة بين الأجهزة الدوائية والسماح بتنظيم فعال ومتسق. علاوة على ذلك، من المتوقع أن تقوم شركات الأدوية متعددة الجنسيات بإنشاء أدوية وأجهزة سريرية بمعدل غير مسبوق لتلبية الطلب على أنظمة توصيل الأدوية المحسنة.
الوصول إلى التقرير الكامل @ https://www.databridgemarketresearch.com/reports/global-drug-device-combination-market
تحلل أبحاث سوق جسر البيانات أن سوق الجمع بين الأدوية والأجهزة سينمو بمعدل نمو سنوي مركب قدره 10.20٪ في الفترة المتوقعة من 2021 إلى 2028 ومن المتوقع أن يصل إلى 30,854.85 مليون دولار أمريكي بحلول عام 2028 من 14,186.35 مليون دولار أمريكي في عام 2020. إن سكان العالم يتقدمون في السن، مما يؤدي إلى زيادة في انتشار الأمراض المزمنة. يمكن أن تكون مجموعات الأدوية والأجهزة وسيلة أكثر فعالية لعلاج هذه الأمراض من العلاج الدوائي التقليدي. يمكن تصميم مجموعات الأدوية والأجهزة وفقًا للاحتياجات الفردية للمرضى، مما قد يؤدي إلى نتائج أفضل. يطالب المرضى بشكل متزايد بطرق أكثر ملاءمة وفعالية لعلاج أمراضهم. يمكن أن توفر مجموعات الأدوية والأجهزة طريقة أكثر ملاءمة وفعالية لتناول الدواء.
تزايد انتشار الأمراض المزمنة من المتوقع أن يقود معدل نمو السوق
إن زيادة انتشار الأمراض المزمنة مثل السكري واضطرابات الجهاز التنفسي والسرطان واعتماد تقنيات مبتكرة لتوصيل الأدوية لهذه الأمراض هي التي تقود السوق. وفقًا لـ IDF 2021، هناك حوالي 537 مليون بالغ مصاب بالسكري، ومن المتوقع أن يرتفع عدد البالغين المصابين بالسكري إلى 643 مليونًا بحلول عام 2030 و783 مليونًا بحلول عام 2045. وبسبب الاستخدام المتزايد لمنتجات توصيل الأدوية المركبة في توصيل الأدوية بشكل موحد، ومن المرجح أن تؤدي مثل هذه الزيادة في الأمراض الأيضية إلى زيادة توسع السوق. علاوة على ذلك، فإن التطبيقات المتزايدة للسلع المركبة بين الأدوية والأجهزة وإدخال منتجات جديدة خلال الفترة المتوقعة من المرجح أن تؤدي إلى توسع السوق.
نطاق التقرير وتقسيم السوق
|
تقرير المقياس
|
تفاصيل
|
|
فترة التنبؤ
|
2021 إلى 2028
|
|
سنة الأساس
|
2020
|
|
سنوات تاريخية
|
2019 (قابل للتخصيص إلى 2013-2018)
|
|
الوحدات الكمية
|
الإيرادات بمليون دولار أمريكي، الأحجام بالوحدات، التسعير بالدولار الأمريكي
|
|
القطاعات المغطاة
|
المنتج (الحاقن التلقائي، التصحيح بالإبر الدقيقة، حبوب منع الحمل الرقمية، جهاز الاستنشاق الذكي، الهلاميات المائية لتوصيل الأدوية، العدسات المستخرجة للدواء وغيرها)، نوع التطبيق (أمراض العظام، أمراض الجهاز التنفسي، مرض السكري، الأورام، أمراض القلب والأوعية الدموية، وغيرها)، المستخدم النهائي ( العيادات، المستشفيات، أماكن الرعاية المنزلية، مراكز الرعاية المتنقلة وغيرها)، قناة التوزيع (العطاء المباشر، مبيعات التجزئة وغيرها)
|
|
البلدان المشمولة
|
الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية وألمانيا وفرنسا والمملكة المتحدة وهولندا وسويسرا وبلجيكا وروسيا وإيطاليا وإسبانيا وتركيا وبقية أوروبا في أوروبا والصين واليابان والهند وكوريا الجنوبية وسنغافورة وماليزيا وأستراليا، تايلاند، إندونيسيا، الفلبين، بقية دول آسيا والمحيط الهادئ (APAC) في منطقة آسيا والمحيط الهادئ (APAC)، المملكة العربية السعودية، الإمارات العربية المتحدة، جنوب أفريقيا، مصر، إسرائيل، وبقية دول الشرق الأوسط وأفريقيا (MEA) كجزء من الشرق الأوسط وأفريقيا (MEA) والبرازيل والأرجنتين وبقية أمريكا الجنوبية كجزء من أمريكا الجنوبية
|
|
تغطية لاعبي السوق
|
Medtronic (أيرلندا)، Zimmer Biomet (الولايات المتحدة)، Amgen Inc. (الولايات المتحدة)، Mediprint (الولايات المتحدة)، Propeller Health (الولايات المتحدة)، BD (الولايات المتحدة)، YPSOMED (سويسرا)، Vaxess Technologies Inc. (الولايات المتحدة)، Subcuject Aps ( الدنمارك)، OcuMedic (الولايات المتحدة)، GlaxoSmithKline plc (المملكة المتحدة)، Bayer AG (ألمانيا)، NanoPass (إسرائيل)، Sensirion AG (سويسرا)، Janssen Pharmaceuticals, Inc. (الولايات المتحدة)، Insulet Corporation (الولايات المتحدة)، Otsuka America Pharmaceutical، Inc. (الولايات المتحدة)، وCGbio (كوريا الجنوبية)
|
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى رؤى السوق مثل القيمة السوقية ومعدل النمو وقطاعات السوق والتغطية الجغرافية واللاعبين في السوق وسيناريو السوق، يتضمن تقرير السوق الذي أعده فريق أبحاث السوق Data Bridge تحليلاً متعمقًا للخبراء وبائيات المرضى وتحليل خطوط الأنابيب. وتحليل الأسعار والإطار التنظيمي
|
تحليل القطاع:
يتم تصنيف السوق العالمية لمجموعة الأدوية والأجهزة إلى أربعة قطاعات بارزة تعتمد على المنتج والتطبيق والمستخدم النهائي وقناة التوزيع.
- على أساس المنتج، يتم تقسيم السوق العالمية لمجموعة الأدوية والأجهزة إلى حاقن تلقائي، ورقعة إبر دقيقة، وحبوب رقمية، وأجهزة استنشاق ذكية، وهلام مائي لتوصيل الأدوية، وعدسات إزالة الدواء، وغيرها. من المتوقع أن يهيمن الحاقن التلقائي على السوق العالمية لمجموعة الأدوية والأجهزة بحصة سوقية تبلغ 55.7٪ مع تزايد الطلب على الأجهزة الطبية في جميع أنحاء العالم لعلاج المرضى.
- على أساس التطبيق، يتم تقسيم السوق العالمية لمجموعة الأدوية والأجهزة إلى أمراض العظام، وأمراض الجهاز التنفسي، والسكري، والأورام، وأمراض القلب والأوعية الدموية، وغيرها. من المتوقع أن تهيمن أمراض العظام على السوق العالمية لتركيبات الأدوية والأجهزة بحصة سوقية تبلغ 48.0٪ بسبب ارتفاع عدد كبار السن وزيادة الطلب على الأجهزة الطبية لتقويم العظام في جميع أنحاء العالم لعلاج المرضى الذين يعانون من أمراض العظام.
- على أساس المستخدم النهائي، يتم تقسيم السوق العالمية لتجميع الأدوية والأجهزة إلى عيادات ومستشفيات وأماكن الرعاية المنزلية ومراكز الرعاية المتنقلة وغيرها. من المتوقع أن تهيمن العيادات على السوق العالمية لتركيبات الأدوية والأجهزة بحصة سوقية تبلغ 41.7% حيث يمكنها تشخيص المرض والحصول على علاج فعال أطول مدة بناءً على نصيحة الأطباء الخبراء في دولة نامية.
سيهيمن قطاع العيادات على قطاع المستخدم النهائي في سوق مجموعة الأدوية والأجهزة
سوف يظهر قطاع العيادات باعتباره شريحة المستخدم النهائي المهيمنة. ويرجع ذلك إلى العدد المتزايد من العيادات في السوق وخاصة في الاقتصادات النامية. علاوة على ذلك، فإن النمو والتوسع في خدمات تطوير الأبحاث على نطاق عالمي سيعزز نمو هذا القطاع.
- على أساس قناة التوزيع، يتم تقسيم السوق العالمية لمجموعة الأدوية والأجهزة إلى العطاءات المباشرة ومبيعات التجزئة وغيرها. من المتوقع أن تهيمن المناقصة المباشرة على السوق العالمية لمجموعة الأدوية والأجهزة بحصة سوقية تبلغ 79.4٪ لأنها توفر منتجات رعاية صحية عالية الجودة وسهلة المنال. بالإضافة إلى ذلك، يوفر العطاء المباشر القيمة الأكثر تنافسية من السوق.
سيهيمن قطاع العطاء المباشر على قطاع قنوات التوزيع في سوق مجموعة الأدوية والأجهزة
سوف يظهر قطاع العطاء المباشر باعتباره الجزء المهيمن ضمن قناة التوزيع. ويرجع ذلك إلى العدد المتزايد من أنشطة تطوير البنية التحتية في السوق خاصة في الاقتصادات النامية. علاوة على ذلك، فإن النمو والتوسع في صناعة الرعاية الصحية في جميع أنحاء العالم سيعزز نمو هذا القطاع.
اللاعبين الرئيسيين
تعترف Data Bridge Market Research بالشركات التالية باعتبارها اللاعبين الرئيسيين في السوق: Medtronic (أيرلندا)، Zimmer Biomet (الولايات المتحدة)، Amgen Inc. (الولايات المتحدة)، Mediprint (الولايات المتحدة)، Propeller Health (الولايات المتحدة)، BD (الولايات المتحدة)، YPSOMED ( سويسرا)، Vaxess Technologies Inc. (الولايات المتحدة)، Subcuject Aps (الدنمارك)، OcuMedic (الولايات المتحدة)، GlaxoSmithKline plc (المملكة المتحدة)، Bayer AG (ألمانيا)، NanoPass (إسرائيل)، Sensirion AG (سويسرا)، Janssen Pharmaceuticals، Inc. (الولايات المتحدة)، وInsulet Corporation (الولايات المتحدة)، وOtsuka America Pharmaceutical, Inc. (الولايات المتحدة)، وCGbio (كوريا الجنوبية).
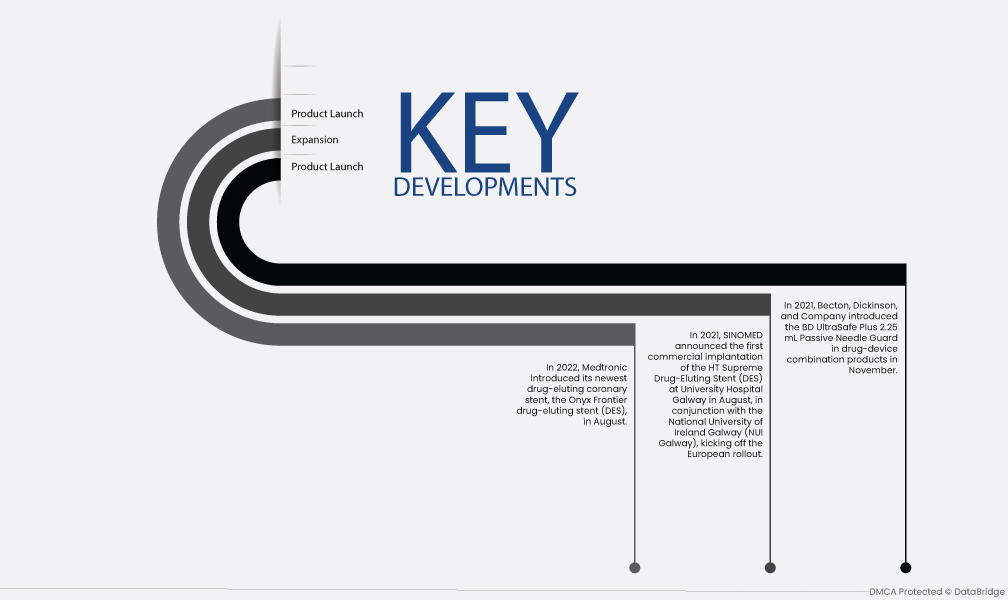
تطوير السوق
- في نوفمبر 2021، قدمت شركة Becton, Dickinson, and Company واقي الإبرة السلبي BD UltraSafe Plus سعة 2.25 مل في منتجات مجموعة الأجهزة الدوائية. يسمح نظام BD UltraSafe Plus سعة 2.25 مل، عند إقرانه بحقنة قابلة للتعبئة مسبقًا، بتوصيل المحاليل البيولوجية تحت الجلد بلزوجة تصل إلى 30 سنتي بواز وأحجام تعبئة تصل إلى 2 مل.
- في أغسطس 2021، أعلنت SINOMED عن أول عملية زرع تجاري لدعامة HT Supreme لإخراج الأدوية (DES) في مستشفى جامعة غالواي بالتعاون مع جامعة أيرلندا الوطنية غالواي (NUI Galway)، لبدء الطرح الأوروبي. سيساعد هذا على الأرجح أطباء القلب التداخليين في علاج المرضى الذين يعانون من مرض الشريان التاجي (CAD) والذين لديهم شرايين ضيقة لا يمكن علاجها عمومًا أثناء التدخل التاجي عن طريق الجلد (PCI) باستخدام تقنية الدعامات الأكبر حجمًا.
- في أغسطس 2022، طرحت شركة Medtronic أحدث دعامة تاجية لتصفية الدواء، وهي دعامة Onyx Frontier لتصفية الدواء (DES). يجمع نظام DES بين منصة الدعامات الأفضل في فئتها وآلية التسليم المحسنة لتحسين إمكانية التسليم والأداء الحاد لدى المرضى الأكثر صعوبة.
التحليل الإقليمي
جغرافيًا، الدول المشمولة في تقرير السوق هي الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية وألمانيا وفرنسا والمملكة المتحدة وهولندا وسويسرا وبلجيكا وروسيا وإيطاليا وإسبانيا وتركيا وبقية أوروبا في أوروبا والصين واليابان والهند. وكوريا الجنوبية وسنغافورة وماليزيا وأستراليا وتايلاند وإندونيسيا والفلبين وبقية دول آسيا والمحيط الهادئ (APAC) والمملكة العربية السعودية والإمارات العربية المتحدة وجنوب إفريقيا ومصر وإسرائيل وبقية الشرق الأوسط و أفريقيا (MEA) كجزء من الشرق الأوسط وأفريقيا (MEA)، والبرازيل والأرجنتين وبقية أمريكا الجنوبية كجزء من أمريكا الجنوبية.
وفقًا لتحليل أبحاث سوق Data Bridge:
أمريكا الشمالية هي المنطقة المهيمنة في سوق مجموعة الأدوية والأجهزة خلال الفترة المتوقعة 2021-2028
ومن المتوقع أن تستمر أمريكا الشمالية في الهيمنة على السوق العالمية في السنوات المقبلة بسبب ارتفاع معدل انتشار الحالات المزمنة، والبنية التحتية الراسخة للرعاية الصحية، وزيادة الوعي بمجموعات الأدوية والأجهزة.
آسيا والمحيط الهادئ من المقدر أن تكون المنطقة الأسرع نموًا في مزيج من المخدرات والأجهزة سوق في الفترة المتوقعة 2021-2028
تعد منطقة آسيا والمحيط الهادئ السوق الأسرع نموًا لمجموعات الأدوية والأجهزة بسبب العدد الكبير من السكان، وزيادة اعتماد التقنيات الجديدة، وزيادة الطلب على الطب الشخصي، وارتفاع معدل الإصابة بالأمراض المزمنة.
للحصول على معلومات أكثر تفصيلاً حول سوق مجموعة الأدوية والأجهزة التقرير اضغط هنا – https://www.databridgemarketresearch.com/reports/global-drug-device-combination-market