Middle East And Africa Hereditary Transthyretin Amyloidosis Market
Taille du marché en milliards USD
TCAC :
% 
| 2023 –2030 | |
| Dollars américains 65,985.45 | |
|
|
|
>Marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique, par diagnostic et traitement (diagnostic et traitement), variation génétique (V122L, T60A, V30M et autres), sexe (homme et femme), indication ( cardiomyopathie (ATTR-CM), polyneuropathie (ATTR-PN) et indications mixtes), utilisateur final (hôpitaux et cliniques, laboratoires de diagnostic, centres de radiologie, instituts universitaires et de recherche, centres de chirurgie ambulatoire et soins à domicile), canal de distribution (appel d'offres direct, distributeurs tiers, pharmacie hospitalière, pharmacie de détail et autres) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et taille du marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique
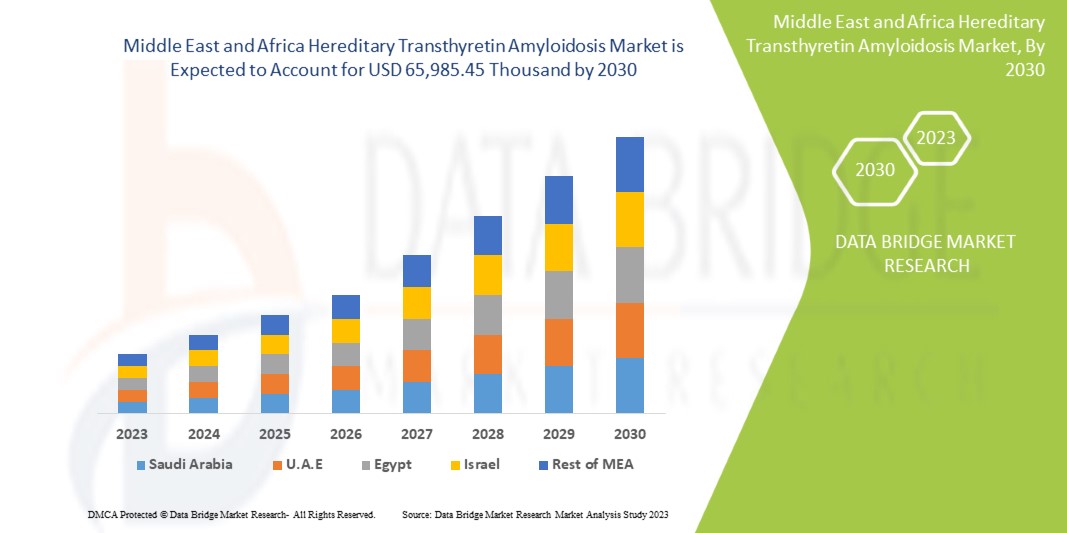
Le marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique devrait croître au cours de l'année de prévision en raison de l'augmentation du nombre d'acteurs du marché et de la disponibilité d'appareils de diagnostic technologiques avancés. Le développement croissant dans le domaine des techniques avancées devrait en outre stimuler la croissance du marché. Cependant, des difficultés telles que les réglementations strictes pour la production et la commercialisation de médicaments de traitement et de dispositifs médicaux pour le diagnostic et la chirurgie devraient freiner la croissance du marché au cours de la période de prévision.
Data Bridge Market Research analyse que le marché de l'amylose héréditaire à transthyrétine au Moyen-Orient et en Afrique devrait atteindre la valeur de 65 985,45 milliers USD d'ici 2030, à un TCAC de 3,1 % au cours de la période de prévision. Ce rapport de marché couvre également en profondeur l'analyse des prix et les avancées technologiques.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable de 2015 à 2020) |
|
Unités quantitatives |
Chiffre d'affaires en milliers de dollars américains, volumes en unités, prix en dollars américains |
|
Segments couverts |
Diagnostic et traitement (Diagnostic et traitement), variation génétique (V122L, T60A, V30M et autres), sexe (homme et femme), indication (cardiomyopathie (ATTR-CM), polyneuropathie (ATTR-PN) et indications mixtes), utilisateur final (hôpitaux et cliniques, laboratoires de diagnostic, centres de radiologie, instituts universitaires et de recherche, centres de chirurgie ambulatoire et soins à domicile), canal de distribution (appel d'offres direct, distributeurs tiers, pharmacie hospitalière, pharmacie de détail et autres) |
|
Pays couverts |
Afrique du Sud, Arabie saoudite, Émirats arabes unis, Égypte, Israël et reste du Moyen-Orient et de l'Afrique |
|
Acteurs du marché couverts |
Pfizer Inc., GE HealthCare, Siemens Healthcare GmbH, Koninklijke Philips NV, CANON MEDICAL SYSTEMS CORPORATION, Neusoft Corporation, NIHON KOHDEN CORPORATION., Shimadzu Corporation, Alnylam Pharmaceuticals, Inc., SCHILLER, Novo Nordisk A/S, FONAR Corp et MinFound Medical Systems Co., entre autres |
Définition du marché
The Middle East and Africa hereditary transthyretin amyloidosis market is a pharmaceutical and biotechnology market focused on the treatment and diagnosis of hATTR, a rare genetic disease characterized by the accumulation of abnormal transthyretin protein in tissues and organs, leading to various organ dysfunctions.
Hereditary transthyretin amyloidosis (hATTR) is a rare, inherited genetic disorder characterized by the accumulation of abnormal transthyretin protein (TTR) in various tissues and organs of the body. TTR is a protein primarily produced in the liver and is responsible for transporting thyroxine (a thyroid hormone) and retinol (vitamin A) in the bloodstream. In hATTR, a genetic mutation leads to the misfolding of TTR proteins, causing them to form amyloid fibrils that deposit in organs, disrupting their normal function. There are different forms of hATTR, including hereditary ATTR polyneuropathy (hATTR-PN) and hereditary ATTR cardiomyopathy (hATTR-CM). In hATTR-PN, the peripheral nerves are primarily affected, resulting in sensory, motor, and autonomic dysfunction. Patients may experience symptoms such as numbness, tingling, muscle weakness, and gastrointestinal problems.
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
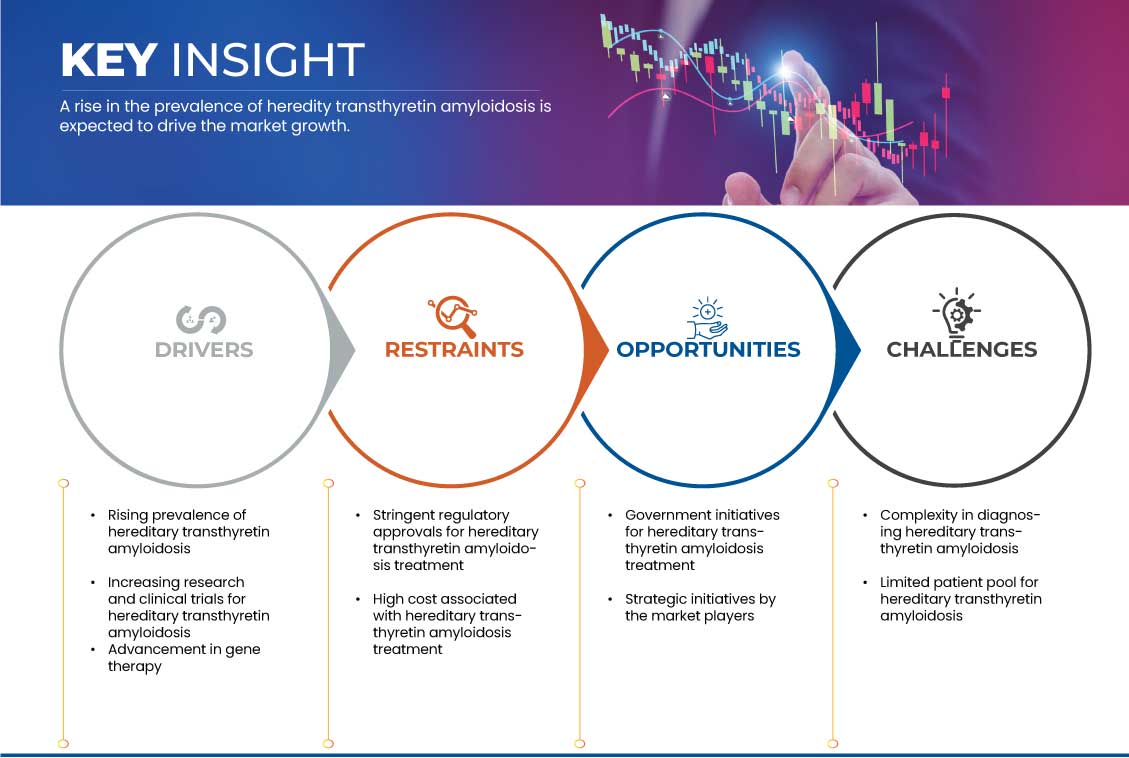
Drivers
- Rising Prevalence of Hereditary Transthyretin Amyloidosis
Hereditary transthyretin amyloidosis (hATTR), also known as familial amyloid polyneuropathy (FAP), is a rare and inherited disorder characterized by the abnormal accumulation of amyloid fibrils in various organs and tissues of the body. Amyloid fibrils are misfolded proteins that clump together and can lead to organ dysfunction over time.
Transthyretin (TTR) is a protein primarily produced by the liver, and its normal function is to transport thyroxine (a thyroid hormone) and retinol (a form of vitamin A) in the blood. In hATTR, a genetic mutation causes the transthyretin protein to become unstable and more likely to misfold, leading to the formation of amyloid deposits.
The prevalence of hereditary transthyretin amyloidosis (hATTR) is on the rise due to various factors. Firstly, improved genetic testing methods have made it easier and more affordable to identify individuals at risk for hATTR, leading to earlier diagnoses and a better understanding of the disease's prevalence. Increased awareness of hATTR among medical professionals and the general public has resulted in more cases being recognized and diagnosed. Better reporting and data collection lead to a more accurate estimation of the prevalence of hATTR is emerging.
Lastly, the growing aging population has likely played a role in the increasing prevalence of hATTR. This condition typically has a later onset, and as the population ages, a larger number of individuals may develop symptoms of hATTR, leading to a rise in reported cases.
- Increasing Research and Clinical Trials for Hereditary Transthyretin Amyloidosis
There is an increase in research and clinical trials focused on hereditary transthyretin amyloidosis (hATTR) for the past few years. This surge in scientific interest can be attributed to several factors, including advancements in our understanding of the disease's genetic basis and pathophysiology. Researchers have identified various mutations in the transthyretin gene associated with hATTR, and this genetic knowledge has paved the way for targeted therapies.
One of the most promising therapeutic approaches for hATTR is the use of TTR stabilizers. These medications aim to stabilize the transthyretin protein, preventing it from misfolding and forming amyloid fibrils. In clinical trials, these drugs have shown promising results in slowing disease progression and improving patients' quality of life.
Another exciting avenue of research for hATTR is gene silencing therapy, specifically RNA interference (RNAi) and antisense oligonucleotides (ASOs). These treatments work by reducing the production of abnormal transthyretin protein, thereby reducing the amount of amyloid fibrils formed. Early clinical trials have shown encouraging results, with some gene silencing therapies demonstrating significant reductions in the levels of abnormal TTR protein in the blood.
In addition to these approaches, gene editing techniques, such as CRISPR-Cas9 are being explored for their potential in treating hATTR. CRISPR-Cas9 allows scientists to precisely edit the transthyretin gene, correcting or removing the disease-causing mutations. Although still in the early stages of development, gene editing holds promise for providing a more permanent and curative solution for hATTR.
Many countries have established patient registries to support research and clinical trials, that collect data on individuals with hATTR. These registries help researchers better understand the natural history of the disease, identify potential participants for clinical trials and track treatment outcomes.
Thus increasing research and clinical trials for hereditary transthyretin amyloidosis is expected to act as a driver for market growth.
Opportunity
Government Initiatives for Hereditary Transthyretin Amyloidosis Treatment
Increasing recognition by governmental bodies for the importance of addressing hereditary transthyretin amyloidosis (hATTR) and providing better support for affected individuals. These governmental initiatives are driven by the understanding that hATTR is a rare and severe genetic disease with a significant impact on patients' lives. The initiatives aim to improve awareness, research, patient care, and access to effective treatments for hATTR.
One crucial aspect of governmental initiatives is research funding. Governments have allocated funds to support scientific research on hATTR, both at the basic science level and for clinical studies. These funds encourage scientists and medical researchers to explore the disease's underlying mechanisms, identify potential therapeutic targets, and develop novel treatment approaches. Governments contribute to advancing knowledge about hATTR and potentially finding better ways to manage and treat the disease by providing financial support to research projects.
Thus, government initiatives for hereditary transthyretin amyloidosis treatment are expected to act as opportunities for market growth.
Restraints / Challenges
- High Cost Associated with Hereditary Transthyretin Amyloidosis Treatment
The high cost associated with hereditary transthyretin amyloidosis (hATTR) treatments can be a significant barrier for patients and healthcare systems.
Several factors contribute to the elevated expenses in developing and providing these therapies. Firstly, research and development (R&D) costs are a major driver of high treatment expenses. Developing new drugs, especially for rare diseases, such as hATTR, requires substantial investments in basic research, preclinical studies, and clinical trials. The cost of identifying potential drug targets, conducting animal studies, and moving through multiple phases of clinical trials can be substantial. Moreover, the risk of failure in the early stages of drug development means that companies must recoup the costs of both successful and unsuccessful ventures, further increasing the financial burden.
Thus, the high cost associated with hereditary transthyretin amyloidosis is expected to act as a restraint for market growth.
- Complexity in Diagnosing Hereditary Transthyretin Amyloidosis
The diagnosis of complex hereditary transthyretin amyloidosis (hATTR) can be a challenging and complex process due to the diverse clinical manifestations and the rarity of the disease. hATTR is a genetically heterogeneous disorder caused by mutations in the transthyretin (TTR) gene, leading to the accumulation of abnormal TTR protein as amyloid deposits in various organs, including the peripheral nerves, heart, and gastrointestinal system.
One of the critical steps in hATTR diagnosis is genetic testing to identify the specific mutation in the TTR gene. However, there are hundreds of known TTR gene mutations, and identifying the exact mutation can be challenging, requiring specialized genetic testing and expertise. Genetic testing is especially crucial in differentiating hATTR from other forms of amyloidosis, such as wild-type ATTR (ATTRwt) and AL amyloidosis, which may have overlapping clinical features.
Thus, complex hereditary transthyretin amyloidosis diagnosis is expected to act as a challenge for market growth.
Recent Development
- In June 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for AMVUTTRA (vutrisiran) for the treatment of Polyneuropathy of hereditary transthyretin-mediated amyloidosis.
- In April 2023, Canon Medical Systems Corporation announced that it had begun clinical research by employing a next-generation X-ray CT system with photon counting computed tomography with the National Cancer Center Japan (NCC) . This will help the organization in developing its products category.
- In December 2022, a new technology AIR Recon DL was recently recognized in the “Best of What's New” awards by Popular Science magazine. It is a new technique developed by GE Healthcare for delivering high-quality images in a short amount of time. AIR Recon DL uses deep-learning technology to simultaneously improve MRI image quality and enable reduced scan time, improving the patient experience.
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Scope
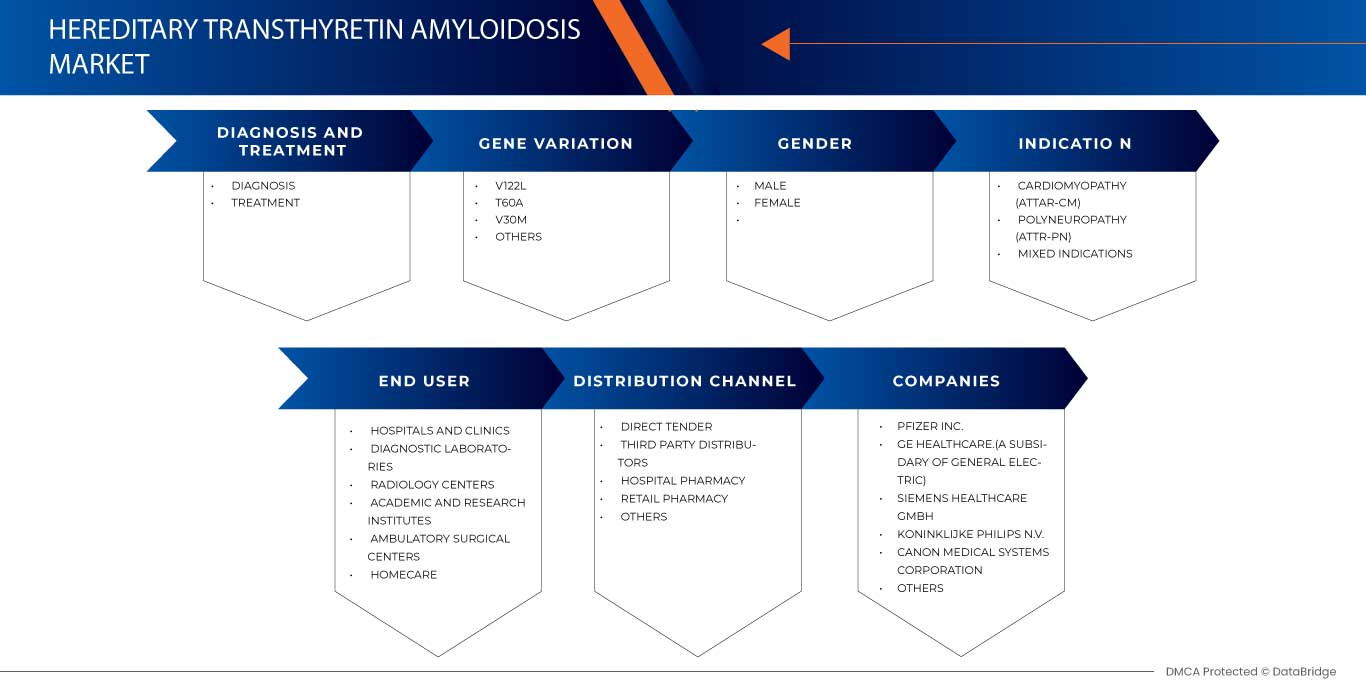
The Middle East and Africa hereditary transthyretin amyloidosis market is segmented into six notable segments, diagnosis and treatment, gene variation, gender, indication, end-user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Diagnosis and Treatment
- Diagnosis
- Treatment
On the basis of diagnosis and treatment, the market is segmented into diagnosis and treatment.
Gene Variation
- V122L
- T60A
- V30M
- Others
On the basis of gene variation, the market is segmented into V122L, T60A, V30M, and others.
Gender
- Male
- Female
On the basis of gender, the market is segmented into male and female.
Indication
- Cardiomyopathy (ATTR-CM)
- Polyneuropathy (ATTR-PN)
- Mixed Indications
On the basis of indications, the market is segmented into cardiomyopathy (ATTR-CM), polyneuropathy (ATTR-PN), and mixed indications.
End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Radiology Centers
- Academic and Research Institutes
- Ambulatory Surgical Centers
- Homecare
On the basis of end user, the market is segmented into hospitals and clinics, diagnostic laboratories, radiology centers, academic and research institutes, ambulatory surgical centers, and homecare.
Distribution Channel
- Direct Tender
- Third party Distributors
- Pharmacie de l'hôpital
- Pharmacie de détail
- Autres
Sur la base du canal de distribution, le marché est segmenté en appels d'offres directs, distributeurs tiers, pharmacies hospitalières, pharmacies de détail et autres.
Analyse/perspectives du marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique
Le marché de l’amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique est analysé et des informations et tendances sur la taille du marché sont fournies par diagnostic et traitement, variation génétique, sexe, indication, utilisateur final et canal de distribution comme référencé ci-dessus.
Les pays couverts par le marché de l’amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique sont l’Afrique du Sud, l’Arabie saoudite, les Émirats arabes unis, l’Égypte, Israël et le reste du Moyen-Orient et de l’Afrique.
L'Afrique du Sud devrait dominer le marché et connaître la croissance la plus rapide en raison de l'augmentation de la sensibilisation et de l'éducation aux maladies parmi les professionnels de la santé et le public, ce qui a probablement contribué à l'identification et à la gestion de maladies telles que l'amylose à transthyrétine héréditaire.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces du porteur et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour chaque pays. En outre, la présence et la disponibilité des marques régionales et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des tarifs nationaux et les routes commerciales sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique
Le paysage concurrentiel du marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique fournit des détails sur le concurrent. Les détails inclus sont un aperçu de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les nouvelles initiatives du marché, la présence régionale, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises par rapport au marché.
Français Certains des principaux acteurs du marché opérant sur le marché de l'amylose à transthyrétine héréditaire au Moyen-Orient et en Afrique sont Pfizer Inc, GE HealthCare, Siemens Healthcare GmbH, Koninklijke Philips NV, CANON MEDICAL SYSTEMS CORPORATION, Neusoft Corporation, NIHON KOHDEN CORPORATION., Shimadzu Corporation, Alnylam Pharmaceuticals, Inc., SCHILLER, Novo Nordisk A/S, FONAR Corp. et MinFound Medical Systems Co., entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 DIAGNOSIS AND TREATMENT LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END-USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S 5 FORCES
4.3 PIPELINE ANALYSIS
5 REGULATORY SCENARIO
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF HEREDITARY TRANSTHYRETIN AMYLOIDOSIS
6.1.2 INCREASING RESEARCH AND CLINICAL TRIALS FOR HEREDITARY TRANSTHYRETIN AMYLOIDOSIS
6.1.3 ADVANCEMENT IN GENE THERAPY
6.2 RESTRAINTS
6.2.1 STRINGENT REGULATORY APPROVALS FOR HEREDITARY TRANSTHYRETIN AMYLOIDOSIS TREATMENT
6.2.2 HIGH COST ASSOCIATED WITH HEREDITARY TRANSTHYRETIN AMYLOIDOSIS TREATMENT
6.3 OPPORTUNITIES
6.3.1 GOVERNMENT INITIATIVES FOR HEREDITARY TRANSTHYRETIN AMYLOIDOSIS TREATMENT
6.3.2 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.4 CHALLENGES
6.4.1 COMPLEXITY IN DIAGNOSING HEREDITARY TRANSTHYRETIN AMYLOIDOSIS
6.4.2 LIMITED PATIENT POOL FOR HEREDITARY TRANSTHYRETIN AMYLOIDOSIS
7 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET : BY COUNTRIES
8 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, COMPANY LANDSCAPE
8.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
9 SWOT ANALYSIS
10 COMPANY PROFILE
10.1 PFIZER INC.
10.1.1 COMPANY SNAPSHOT
10.1.2 REVENUE ANALYSIS
10.1.3 PRODUCT PORTFOLIO
10.1.4 RECENT DEVELOPMENT
10.2 GE HEALTHCARE.( A SUBSIDARY OF GENERAL ELECTRIC)
10.2.1 COMPANY SNAPSHOT
10.2.2 REVENUE ANALYSIS
10.2.3 PRODUCT PORTFOLIO
10.2.4 RECENT DEVELOPMENT
10.3 SIEMENS HEALTHCARE GMBH
10.3.1 COMPANY SNAPSHOT
10.3.2 REVENUE ANALYSIS
10.3.3 PRODUCT PORTFOLIO
10.3.4 RECENT DEVELOPMENT
10.4 KONINKLIJKE PHILIPS N.V.
10.4.1 COMPANY SNAPSHOT
10.4.2 REVENUE ANALYSIS
10.4.3 PRODUCT PORTFOLIO
10.4.4 RECENT DEVELOPMENT
10.5 CANON MEDICAL SYSTEMS CORPORATION
10.5.1 COMPANY SNAPSHOT
10.5.2 REVENUE ANALYSIS
10.5.3 PRODUCT PORTFOLIO
10.5.4 RECENT DEVELOPMENT
10.6 ALNYLAM PHARMACEUTICALS, INC.
10.6.1 COMPANY SNAPSHOT
10.6.2 REVENUE ANALYSIS
10.6.3 PRODUCT PORTFOLIO
10.6.4 RECENT DEVELOPMENT
10.7 ASTRAZENECA
10.7.1 COMPANY SNAPSHOT
10.7.2 REVENUE ANALYSIS
10.7.3 PRODUCT PORTFOLIO
10.7.4 RECENT DEVELOPMENT
10.8 FONAR CORP.
10.8.1 COMPANY SNAPSHOT
10.8.2 REVENUE ANALYSIS
10.8.3 PRODUCT PORTFOLIO
10.8.4 RECENT DEVELOPMENT
10.9 MINFOUND MEDICAL SYSTEMS CO.,
10.9.1 COMPANY SNAPSHOT
10.9.2 PRODUCT PORTFOLIO
10.9.3 RECENT DEVELOPMENT
10.1 NEUSOFT CORPORATION
10.10.1 COMPANY SNAPSHOT
10.10.2 REVENUE ANALYSIS
10.10.3 PRODUCT PORTFOLIO
10.10.4 RECENT DEVELOPMENT
10.11 NIHON KOHDEN CORPORATION.
10.11.1 COMPANY SNAPSHOT
10.11.2 RECENT FINANCIALS
10.11.3 PRODUCT PORTFOLIO
10.11.4 RECENT DEVELOPMENT
10.12 NOVO NORDISK A/S
10.12.1 COMPANY SNAPSHOT
10.12.2 REVENUE ANALYSIS
10.12.3 PRODUCT PORTFOLIO
10.12.4 RECENT DEVELOPMENT
10.13 SCHILLER
10.13.1 COMPANY SNAPSHOT
10.13.2 PRODUCT PORTFOLIO
10.13.3 RECENT DEVELOPMENT
10.14 SHIMADZU CORPORATION
10.14.1 COMPANY SNAPSHOT
10.14.2 REVENUE ANALYSIS
10.14.3 PRODUCT PORTFOLIO
10.14.4 RECENT DEVELOPMENT
11 QUESTIONNAIRE
12 RELATED REPORTS
Liste des tableaux
TABLE 1 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, PIPELINE ANALYSIS
TABLE 2 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 3 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 4 MIDDLE EAST AND AFRICA DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 5 MIDDLE EAST AND AFRICA IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 MIDDLE EAST AND AFRICA NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 7 MIDDLE EAST AND AFRICA BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 8 MIDDLE EAST AND AFRICA TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 MIDDLE EAST AND AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 10 MIDDLE EAST AND AFRICA APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 11 MIDDLE EAST AND AFRICA PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 12 MIDDLE EAST AND AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 13 MIDDLE EAST AND AFRICA GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 14 MIDDLE EAST AND AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 15 MIDDLE EAST AND AFRICA PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 16 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 17 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 18 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 19 MIDDLE EAST AND AFRICA POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 20 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 21 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 22 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 23 SOUTH AFRICA DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 24 SOUTH AFRICA IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 25 SOUTH AFRICA NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 26 SOUTH AFRICA BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 27 SOUTH AFRICA TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 28 SOUTH AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 29 SOUTH AFRICA APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 30 SOUTH AFRICA PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 31 SOUTH AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 32 SOUTH AFRICA GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 33 SOUTH AFRICA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 34 SOUTH AFRICA PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 35 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 36 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 37 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 38 SOUTH AFRICA POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 39 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 40 SOUTH AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 41 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 42 SAUDI ARABIA DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 43 SAUDI ARABIA IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 44 SAUDI ARABIA NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 45 SAUDI ARABIA BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 46 SAUDI ARABIA TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 47 SAUDI ARABIA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 48 SAUDI ARABIA APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 SAUDI ARABIA PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 SAUDI ARABIA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 51 SAUDI ARABIA GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 52 SAUDI ARABIA MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 53 SAUDI ARABIA PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 54 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 55 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 56 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 57 SAUDI ARABIA POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 58 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 59 SAUDI ARABIA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 60 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 61 U.A.E DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 62 U.A.E IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 63 U.A.E NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 64 U.A.E BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 65 U.A.E TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 U.A.E MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 67 U.A.E APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 U.A.E PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 69 U.A.E MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 70 U.A.E GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 71 U.A.E MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 72 U.A.E PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 73 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 74 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 75 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 76 U.A.E POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 77 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 78 U.A.E HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 79 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 80 EGYPT DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 81 EGYPT IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 82 EGYPT NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 83 EGYPT BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 84 EGYPT TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 85 EGYPT MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 86 EGYPT APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 87 EGYPT PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 88 EGYPT MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 89 EGYPT GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 90 EGYPT MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 91 EGYPT PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 92 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 93 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 94 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 95 EGYPT POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 96 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 97 EGYPT HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 98 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 99 ISRAEL DIAGNOSIS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TESTS, 2021-2030 (USD THOUSAND)
TABLE 100 ISRAEL IMAGING TEST IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 101 ISRAEL NUCLEAR IMAGING IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY IMAGING TEST, 2021-2030 (USD THOUSAND)
TABLE 102 ISRAEL BIOPSY IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 103 ISRAEL TREATMENT IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 104 ISRAEL MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY APPROVAL, 2021-2030 (USD THOUSAND)
TABLE 105 ISRAEL APPROVED MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 106 ISRAEL PIPELINE MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 107 ISRAEL MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY MODE OF ACTION, 2021-2030 (USD THOUSAND)
TABLE 108 ISRAEL GENE SILENCER IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 109 ISRAEL MEDICATIONS IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 110 ISRAEL PARENTERAL IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 111 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENE VARIATION, 2021-2030 (USD THOUSAND)
TABLE 112 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 113 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY INDICATION, 2021-2030 (USD THOUSAND)
TABLE 114 ISRAEL POLYNEUROPATHY (ATTR-PN) IN HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 115 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 116 ISRAEL HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 117 REST OF MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET, BY DIAGNOSIS AND TREATMENT, 2021-2030 (USD THOUSAND)
Liste des figures
FIGURE 1 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: REGIONAL VS COUNTRY MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: MARKET END-USER COVERAGE GRID
FIGURE 9 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: SEGMENTATION
FIGURE 11 RISING PREVALENCE OF HEREDITARY TRANSTHYRETIN AMYLOIDOSIS IS EXPECTED TO DRIVE THE MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET GROWTH IN THE FORECAST PERIOD
FIGURE 12 THE DIAGNOSIS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET
FIGURE 14 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: SNAPSHOT (2022)
FIGURE 15 MIDDLE EAST AND AFRICA HEREDITARY TRANSTHYRETIN AMYLOIDOSIS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.