North America Hla Typing Transplant Market
Market Size in USD Million
CAGR :
% 
 USD
525.44 Million
USD
972.55 Million
2025
2033
USD
525.44 Million
USD
972.55 Million
2025
2033
| 2026 –2033 | |
| USD 525.44 Million | |
| USD 972.55 Million | |
|
|
|
|
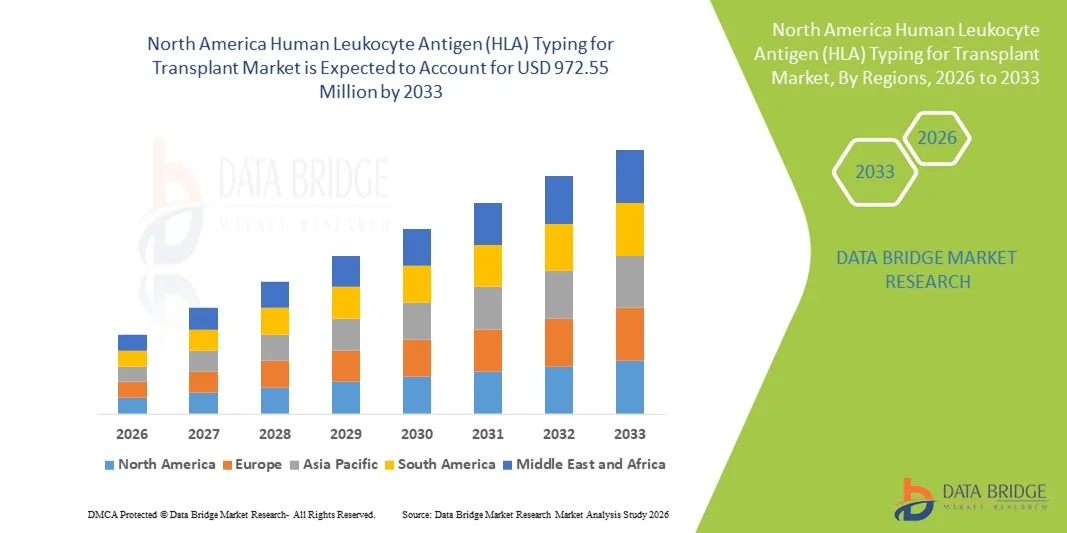
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Size
- The North America Human Leukocyte Antigen (HLA) typing for transplant market size was valued at USD 525.44 million in 2025 and is expected to reach USD 972.55 million by 2033, at a CAGR of 8.0% during the forecast period
- The market growth is largely fueled by the increasing number of organ transplant procedures, rising prevalence of chronic and hematologic diseases, and advancements in molecular and next-generation sequencing technologies for precise donor-recipient matching
- Furthermore, growing awareness of the importance of accurate tissue matching, coupled with supportive healthcare infrastructure and rising demand for personalized transplant solutions, is establishing HLA typing as a critical component of transplant success. These converging factors are accelerating the adoption of HLA typing solutions, thereby significantly boosting the industry's growth
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Analysis
- HLA typing, providing precise tissue matching for organ and stem cell transplants, is becoming a critical component of modern transplant protocols in both clinical and research settings due to its accuracy, rapid turnaround, and integration with advanced diagnostic technologies
- The growing demand for HLA typing is primarily fueled by the rising number of organ and hematopoietic stem cell transplants, increasing prevalence of chronic and genetic disorders, and advancements in molecular and next-generation sequencing-based HLA typing methods
- The United States dominated the North America Human Leukocyte Antigen (HLA) typing market with the largest revenue share of 75.2% in 2025, characterized by advanced healthcare infrastructure, high awareness of transplant success factors, and a strong presence of key industry players
- Canada is expected to be the fastest-growing country in the North America Human Leukocyte Antigen (HLA) typing market during the forecast period due to increasing organ transplant procedures and supportive regulatory frameworks
- Reagents and Consumables segment dominated the North America Human Leukocyte Antigen (HLA) typing market with a share of 43.4% in 2025, driven by its essential role in ensuring accurate, reliable, and high-throughput HLA testing for improved transplant outcomes
Report Scope and North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Segmentation
|
Attributes |
North America Human Leukocyte Antigen (HLA) Typing for Transplant Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Trends
Advancements in High-Resolution and NGS-Based Typing
- A significant and accelerating trend in the North America HLA typing market is the adoption of high-resolution molecular and next-generation sequencing (NGS) technologies, enhancing accuracy and turnaround times for donor-recipient matching
- For instance, the AlloSeq Tx NGS assay integrates high-throughput sequencing with automated data analysis, allowing transplant centers to perform rapid and precise HLA typing for multiple loci simultaneously
- NGS-based HLA typing enables features such as high-resolution allele-level matching and improved detection of rare variants, increasing transplant success rates. For instance, some Immucor NGS solutions provide automated genotyping and advanced reporting to support clinical decision-making
- The seamless integration of NGS and molecular typing platforms with laboratory information systems (LIS) facilitates centralized workflow management, allowing clinicians to track samples, results, and donor databases efficiently
- This trend towards more precise, automated, and high-throughput HLA typing is fundamentally reshaping transplant diagnostics. Consequently, companies such as One Lambda and Thermo Fisher Scientific are developing AI-enabled HLA analysis tools for improved accuracy and faster reporting
- The demand for high-resolution, automated, and NGS-enabled HLA typing solutions is growing rapidly across both hospitals and specialized transplant centers, as clinical outcomes increasingly depend on precise tissue matching
- Adoption of cloud-based HLA typing data management platforms is rising, enabling laboratories to securely store, analyze, and share HLA profiles, improving turnaround time and accessibility for transplant centers. For instance, some AI-powered platforms allow real-time analytics for donor compatibility
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Dynamics
Driver
Rising Demand Due to Increasing Organ and Stem Cell Transplants
- The rising number of organ and hematopoietic stem cell transplants in North America, coupled with the need for accurate donor-recipient matching, is a significant driver for the growing adoption of HLA typing solutions
- For instance, in March 2025, Thermo Fisher Scientific announced the launch of a high-throughput NGS HLA typing solution aimed at improving transplant compatibility and lab workflow efficiency
- As transplant centers prioritize better clinical outcomes, HLA typing offers advanced features such as high-resolution allele matching, automated genotyping, and comprehensive reporting, providing a critical upgrade over conventional serological methods
- Furthermore, the expanding network of transplant centers and increasing awareness among clinicians and patients about the importance of precise tissue matching are making HLA typing an essential component of transplant protocols
- The integration of HLA typing solutions with laboratory information systems, electronic medical records, and donor registries enhances efficiency and accessibility, driving widespread adoption in both public and private healthcare settings
- Government initiatives and funding programs supporting organ and stem cell transplantation are encouraging hospitals to adopt advanced HLA typing solutions. For instance, Medicare reimbursement policies increasingly cover high-resolution HLA testing for eligible patients
- Rising collaborations between diagnostics companies and research institutions are facilitating the development of rapid HLA typing assays and AI-based predictive tools for transplant outcomes. For instance, partnerships with major hospitals help validate new workflows
Restraint/Challenge
High Costs and Regulatory Compliance Challenges
- The relatively high cost of advanced HLA typing systems, including NGS-based platforms, poses a significant challenge to broader market adoption, particularly for smaller laboratories and budget-conscious hospitals
- For instance, high-cost instruments and reagents for NGS HLA typing have limited adoption in some regional and community transplant centers despite their clinical benefits
- Regulatory compliance requirements and the need for validation of new HLA typing assays add further complexity, increasing the time and expense for laboratories to implement advanced solutions. For instance, FDA and CAP guidelines require rigorous testing and documentation for clinical use
- Addressing these challenges through cost-effective solutions, reagent rental programs, and streamlined regulatory support is crucial for encouraging wider adoption. Companies such as Immucor and One Lambda emphasize validated protocols and training programs to assist laboratories in compliance
- While technological advances are reducing costs over time, the initial investment and regulatory hurdles continue to limit adoption, particularly in smaller-scale facilities that cannot easily absorb upfront expenditures
- Limited skilled personnel trained in advanced HLA typing techniques can slow adoption and affect testing turnaround times. For instance, many smaller labs rely on centralized reference laboratories for complex NGS-based typing
- Variability in reimbursement policies across states and healthcare providers can also restrict the accessibility of high-resolution HLA typing. For instance, some centers may delay adoption due to uncertainty in insurance coverage for advanced assays
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Scope
The market is segmented on the basis of products and services, technology, transplant type, application, and end user.
- Products and Services
On the basis of products and services, the market is segmented into reagents and consumables, instruments, and software & services. The Reagents and Consumables segment dominated the market in 2025 with a market share of 43.4% due to its essential role in ensuring accurate and high-throughput HLA typing. Reagents such as primers, probes, and control kits are critical for both molecular and sequencing-based assays, directly impacting test reliability. Consumables such as plates, tubes, and pipette tips ensure smooth laboratory workflows and prevent cross-contamination. High demand from hospitals, transplant centers, and reference laboratories maintains steady consumption of reagents. In addition, suppliers frequently innovate in reagents to enhance sensitivity, specificity, and speed of HLA typing assays. The segment also benefits from recurring revenue opportunities, as consumables are regularly replenished for ongoing testing.
The Instruments segment is expected to witness the fastest growth during forecast period due to increasing adoption of advanced automated HLA typing platforms and NGS-based sequencers. Modern instruments reduce manual labor, enhance throughput, and provide precise high-resolution typing. Rising investment in laboratory infrastructure and growing preference for integrated, automated solutions in transplant centers are boosting instrument sales. Continuous technological upgrades and AI-enabled features in instruments are attracting hospitals and independent laboratories. Demand is further fueled by expanding research applications and high-volume transplant testing workflows. Instruments also offer longer-term returns for labs compared to consumables, driving procurement and expansion plans.
- By Technology
On the basis of technology, the market is segmented into sequencing-based molecular assays, molecular assay technologies, and non-molecular assay technologies. The Sequencing-Based Molecular Assays segment dominated the market in 2025 due to its high resolution and ability to detect rare alleles critical for donor-recipient matching. Sequencing approaches provide precise, allele-level HLA typing and improve transplant success rates. They allow laboratories to perform multiplex testing for multiple loci simultaneously, reducing turnaround time. Advanced platforms automate workflow and minimize human errors, enhancing reliability. The segment also benefits from increasing adoption in hospitals and reference labs focusing on complex transplants. Rising collaborations between HLA assay providers and transplant networks further strengthen this segment’s market dominance.
The Molecular Assay Technologies segment is expected to witness the fastest growth during forecast period as more labs shift from traditional serology to PCR- and NGS-based molecular methods. These assays offer quicker results and higher specificity for both solid organ and stem cell transplants. Adoption is rising due to increasing awareness of clinical benefits, support from reimbursement policies, and enhanced throughput. Molecular assays are increasingly integrated with laboratory information systems to streamline testing. Growth is further driven by investments in precision medicine and personalized transplant protocols. Continuous innovation in reagents and assay kits also accelerates adoption across North America.
- By Transplant Type
On the basis of transplant type, the market is segmented into solid organ transplant and haematopoietic stem cell transplant. The Haematopoietic Stem Cell Transplant segment dominated the market in 2025 due to the critical need for precise HLA matching in bone marrow and stem cell transplants. Incorrect matching can lead to graft-versus-host disease (GVHD), making high-resolution typing mandatory. Stem cell transplant centers require frequent, high-throughput testing for donors and recipients, ensuring sustained demand. Growing prevalence of hematologic cancers and genetic blood disorders increases the volume of tests. Advanced sequencing and molecular typing technologies further support adoption in this segment. Increasing collaborations with donor registries such as Be The Match enhance access to HLA data, strengthening this segment’s market share.
The Solid Organ Transplant segment is expected to witness the fastest growth during forecast period driven by increasing numbers of kidney, liver, heart, and lung transplants across the U.S. and Canada. Rising awareness among clinicians and patients about the importance of HLA matching is expanding adoption in hospitals. Regulatory encouragement and support from organ transplant networks also boost demand. New sequencing-based technologies and faster molecular assays are being deployed in transplant centers, enhancing workflow efficiency. Growth is further driven by the expansion of public and private transplant programs, increasing patient throughput and testing frequency.
- By Application
On the basis of application, the market is segmented into diagnostic applications and research applications. The Diagnostic Applications segment dominated the market in 2025 due to its critical role in pre-transplant donor-recipient compatibility testing. Hospitals and transplant centers rely on accurate HLA typing to reduce transplant failure risk. Diagnostic applications require high-throughput, reproducible results, fueling recurring demand for reagents, consumables, and instruments. The segment benefits from increasing organ transplant procedures and stem cell therapies. Adoption of sequencing-based molecular assays ensures high accuracy and supports personalized treatment decisions. Collaborations between clinical labs and research institutions further strengthen diagnostic adoption.
The Research Applications segment is expected to witness the fastest growth during forecast period due to rising studies on HLA diversity, transplant immunology, and population genetics. Research laboratories and academic institutes increasingly use NGS-based typing for genetic studies and clinical trial validation. Growth is supported by government and private funding for immunogenetics and transplant research. The segment also benefits from advancements in bioinformatics tools, AI-enabled analysis, and cloud-based HLA databases. Collaborative research with hospitals enhances translational applications, accelerating adoption.
- By End User
On the basis of end user, the market is segmented into independent reference laboratories, hospitals and transplant centers, research laboratories and academic institutes. The Hospitals and Transplant Centers segment dominated the market in 2025 due to high testing volumes and the critical need for timely, accurate HLA typing for transplant success. Large hospitals and specialized transplant centers often deploy automated sequencing and molecular platforms for in-house testing. Integration with electronic medical records and patient databases ensures streamlined workflows. The segment benefits from increasing organ and stem cell transplant procedures. Rising adoption of high-resolution typing, AI-enabled analysis, and automated reporting supports dominance. Collaborations with reagent and instrument suppliers further strengthen the segment.
The Independent Reference Laboratories segment is expected to witness the fastest growth during forecast period due to outsourcing trends and increasing demand from smaller hospitals and clinics. Reference labs offer cost-effective, high-throughput HLA typing services without the need for hospitals to invest in expensive instruments. Growth is supported by rapid turnaround times, centralized expertise, and access to high-resolution assays. Expanding partnerships with transplant centers and research institutes further fuel adoption. Regulatory compliance, quality certifications, and integration with laboratory networks also enhance market share.
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Regional Analysis
- The United States dominated the North America Human Leukocyte Antigen (HLA) typing market with the largest revenue share of 75.2% in 2025, characterized by advanced healthcare infrastructure, high awareness of transplant success factors, and a strong presence of key industry players
- Clinicians and transplant centers in the U.S. highly value the accuracy, high-resolution results, and rapid turnaround times offered by modern HLA typing solutions, which are critical for successful donor-recipient matching and improved transplant outcomes
- This strong adoption is further supported by robust reimbursement policies, integration of HLA testing with electronic medical records and laboratory information systems, and ongoing investments in laboratory automation and AI-enabled HLA analysis platforms, establishing HLA typing as a critical diagnostic service across hospitals, specialized transplant centers, and independent reference laboratories
The United States Human Leukocyte Antigen (HLA) Typing for Transplant Market Insight
The United States Human Leukocyte Antigen (HLA) Typing for Transplant market captured the largest revenue share of 75.2% in 2025 within North America, driven by the high number of organ and hematopoietic stem cell transplants and advanced healthcare infrastructure. Hospitals and specialized transplant centers prioritize high-resolution HLA typing for improved donor-recipient matching, minimizing risks such as graft-versus-host disease. The adoption of sequencing-based molecular assays and AI-enabled analysis platforms is rapidly increasing, enhancing accuracy and workflow efficiency. Moreover, the availability of reimbursement policies for HLA testing, along with integration with electronic medical records and laboratory information systems, is further supporting market growth. The growing demand for rapid, high-throughput HLA typing in both clinical and research applications continues to propel the market forward.
Canada Human Leukocyte Antigen (HLA) Typing for Transplant Market Insight
The Canada Human Leukocyte Antigen (HLA) Typing for Transplant market is expected to grow at a substantial CAGR during the forecast period, primarily fueled by increasing organ and stem cell transplant procedures and supportive government initiatives. Canadian transplant centers are adopting high-resolution molecular and sequencing-based HLA typing to improve clinical outcomes and reduce transplant-related complications. The rising awareness among clinicians and patients regarding the importance of precise donor-recipient matching is driving adoption. Laboratories and hospitals are integrating advanced HLA typing platforms with LIS systems to enhance efficiency and reporting. In addition, the expansion of national donor registries and research collaborations supports growth. The increasing focus on precision medicine and personalized transplant protocols is further boosting the market.
Mexico Human Leukocyte Antigen (HLA) Typing for Transplant Market Insight
The Mexico Human Leukocyte Antigen (HLA) Typing for Transplant market is poised to grow at a notable CAGR during the forecast period, driven by the rising number of transplant procedures and improvements in healthcare infrastructure. Hospitals and reference laboratories are increasingly adopting automated and sequencing-based HLA typing solutions to ensure accuracy and reliability in donor matching. Government support for organ donation programs and increased awareness of transplant outcomes are contributing to market expansion. In addition, collaboration with U.S.-based transplant centers and research institutions facilitates knowledge transfer and adoption of advanced HLA testing technologies. The growing demand for cost-effective and high-throughput solutions is stimulating investment in laboratory automation. Efforts to standardize HLA testing and reporting practices across clinical and research applications also support market growth.
North America Human Leukocyte Antigen (HLA) Typing for Transplant Market Share
The North America Human Leukocyte Antigen (HLA) Typing for Transplant industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN (Netherlands)
- Illumina, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- BD (U.S.)
- Luminex Corporation (U.S.)
- CareDx, Inc. (U.S.)
- Immucor, Inc. (U.S.)
- BAG Diagnostics GmbH (Germany)
- inno-train Diagnostik GmbH (Germany)
- CeGaT GmbH (Germany)
- Bionobis (France)
- ProImmune Ltd (U.K.)
- Eurofins Scientific SE (Luxembourg)
- HistoGenetics, Inc. (U.S.)
- Immudex (Denmark)
- GenDx (Netherlands)
- DiaSorin S.p.A. (Italy)
What are the Recent Developments in North America Human Leukocyte Antigen (HLA) Typing for Transplant Market?
- In October 2025, CareDx, Inc. announced the launch of AlloSeq Tx11, an advanced HLA typing solution featuring enhanced Class II coverage to improve donor‑recipient matching for both solid organ and hematopoietic stem cell transplants. The new solution also incorporates non‑HLA markers (such as ABO and CCR5) to broaden transplant risk profiles and reduce the need for retesting, signaling a major evolution in clinical transplant immunogenetics
- In April 2025, Thermo Fisher Scientific introduced the One Lambda™ HybriType™ HLA Plus Typing Flex kit, an NGS hybrid capture assay designed to enhance high‑resolution HLA genotyping with improved read balance and reduced allele dropout, offering laboratories faster, more reliable workflows for research and transplant diagnostics
- In October 2024, CareDx presented significant advancements to its HLA typing portfolio at the American Society for Histocompatibility & Immunogenetics Annual Meeting, including upgraded AlloSeq™ platforms and a newly improved QTYPE rapid HLA typing solution with enhanced resolution for donor matching, highlighting continuous innovation in transplant diagnostics
- In September 2024, Immucor introduced the LIFECODES LifeScreen Deluxe, a high‑resolution HLA antibody screening platform designed to support faster and more accurate donor‑recipient compatibility testing in transplant diagnostics. The new LifeScreen Deluxe expands the company’s existing HLA antibody portfolio, offering enhanced sensitivity and comprehensive class I and class II antigen detection to improve transplant matching and minimize immunologic risk
- In June 2024, Omixon Biocomputing Ltd received In Vitro Diagnostic Regulation (IVDR) approval for its NanoTYPE™ CE IVDR kit, a high‑resolution multiplex 11‑loci HLA amplification assay compatible with Oxford Nanopore sequencing platforms. This regulatory milestone confirmed the quality and clinical readiness of NanoTYPE for detailed HLA genotyping, facilitating faster and more scalable HLA typing workflows in transplant diagnostic laboratories
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.