Global Enzyme Immunoassay Eia Reagents And Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
17.59 Billion
USD
23.40 Billion
2024
2032
USD
17.59 Billion
USD
23.40 Billion
2024
2032
| 2025 –2032 | |
| USD 17.59 Billion | |
| USD 23.40 Billion | |
|
|
|
|
Enzyme Immunoassay (EIA) Reagents and Devices Market Size
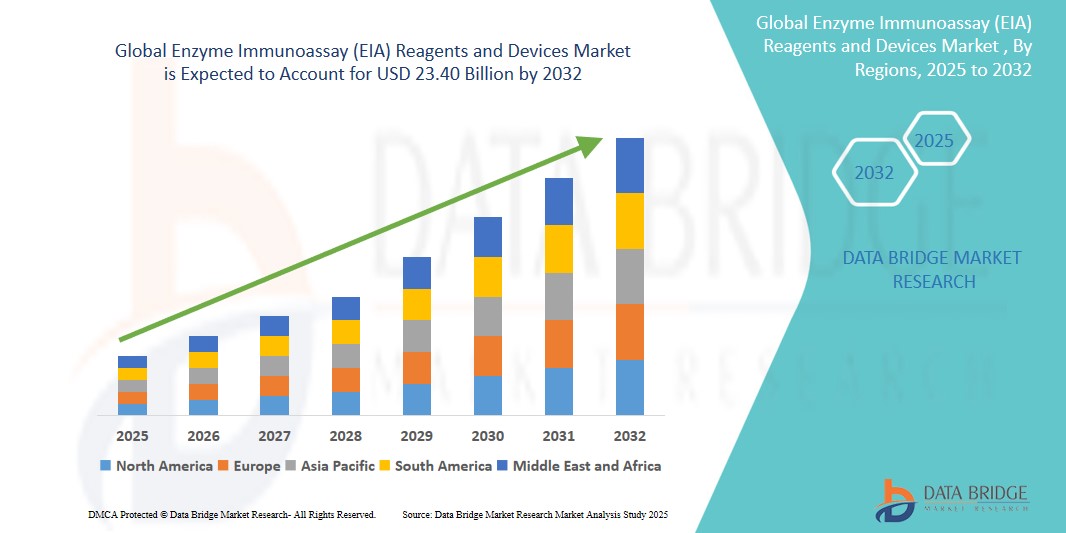
- The global Enzyme Immunoassay (EIA) Reagents and Devices market size was valued at USD 17.59 billion in 2024 and is expected to reach USD 23.40 billion by 2032, at a CAGR of 3.63% during the forecast period
- The market growth is largely fuelled by the increasing prevalence of infectious diseases and chronic conditions, coupled with technological advancements in diagnostic testing, leading to increased adoption of EIAs in various healthcare settings
- Furthermore, rising demand for rapid, accurate, and cost-effective diagnostic solutions is establishing EIAs as a preferred choice for disease detection and screening. These converging factors are accelerating the uptake of EIA reagents and devices, thereby significantly boosting the industry's growth.
Enzyme Immunoassay (EIA) Reagents and Devices Market Analysis
- The Enzyme Immunoassay (EIA) Reagents and Devices Market comprises the products used in Enzyme Immunoassays (EIAs), which are biochemical techniques that detect the presence of a substance, usually an antigen or antibody, in a biological sample. EIAs utilize enzyme-labelled antibodies or antigens to quantify the target substance, making them valuable tools in diagnostics and research.
- The escalating demand for Enzyme Immunoassay (EIA) Reagents and Devices is primarily fueled by the increasing global burden of diseases, growing awareness of early disease detection, and a rising preference for minimally invasive diagnostic techniques.
- North America dominates the Enzyme Immunoassay (EIA) Reagents and Devices market with the largest revenue share of 40.01% in 2025, characterized by well-established healthcare infrastructure, high healthcare expenditure, and a strong presence of key industry players, with the U.S. experiencing substantial growth in EIA Reagents and Devices usage, particularly in clinical diagnostics and research settings, driven by innovations from both established pharmaceutical companies and research institutions focusing on advanced diagnostic assays and automation.
- Asia-Pacific is expected to be the fastest growing region in the Enzyme Immunoassay (EIA) Reagents and Devices market during the forecast period due to increasing prevalence of infectious diseases, expanding healthcare access, and rising healthcare expenditure.
- Enzyme Immunoassays segment is expected to dominate the Enzyme Immunoassay (EIA) Reagents and Devices market with a market share of 43.2% in 2025, driven by its versatility, wide range of applications, and established use in various diagnostic and research settings.
Report Scope and Enzyme Immunoassay (EIA) Reagents and Devices Market Segmentation
|
Attributes |
Enzyme Immunoassay (EIA) Reagents and Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Enzyme Immunoassay (EIA) Reagents and Devices Market Trends
“Technological advancements in EIA assays”
- Enhanced Efficiency Through Automation and Digitalization: A significant and accelerating trend in the Enzyme Immunoassay (EIA) Reagents and Devices market is the increasing automation of EIA workflows and the integration of digital technologies for data management and analysis. This evolution is significantly enhancing laboratory efficiency, reducing turnaround times, and improving the accuracy and reliability of test results.
- For instance, companies are developing automated EIA processors that can handle multiple samples simultaneously, reducing the need for manual intervention and minimizing the risk of errors. Similarly, the integration of software solutions for data analysis and reporting streamlines laboratory operations and facilitates the interpretation of complex results.
- Automation in EIA testing enables features such as high-throughput screening, which is crucial for processing large volumes of samples in clinical diagnostics and research. Digitalization also allows for the seamless transfer of data to Laboratory Information Management Systems (LIMS), improving data integrity and accessibility. Furthermore, the use of robotics and AI in EIA processing can further enhance precision and reproducibility.
- The seamless integration of automated EIA systems with laboratory networks and digital platforms facilitates centralized control over testing processes and enables remote monitoring of equipment and workflows. Through a unified interface, laboratory personnel can manage assays, track samples, and access results from various locations, creating a more efficient and connected laboratory environment.
- This trend towards more automated, digitalized, and interconnected EIA systems is fundamentally reshaping laboratory operations and diagnostic practices. Consequently, companies are investing in the development of advanced automation solutions and software platforms to meet the evolving needs of clinical and research laboratories.
- The demand for EIA solutions that offer seamless automation and digital integration is growing rapidly across both clinical and research settings, as laboratories increasingly prioritize efficiency, accuracy, and data management capabilities.
Enzyme Immunoassay (EIA) Reagents and Devices Market Dynamics
Driver
“Rising prevalence of infectious and chronic diseases”
- Growing Need Due to Rising Disease Prevalence and Diagnostic Demand: The increasing prevalence of infectious diseases and chronic conditions, coupled with the growing demand for accurate and timely diagnosis, is a significant driver for the heightened demand for EIA reagents and devices.
- For instance, the ongoing need for effective diagnostic tools for diseases like HIV, hepatitis, and various cancers, alongside the emergence of new infectious agents, fuels the demand for EIAs. Similarly, the rise in chronic diseases, such as autoimmune disorders and endocrine disorders, necessitates reliable and sensitive diagnostic methods like EIAs.
- As healthcare systems worldwide focus on early disease detection, preventive care, and personalized medicine, the role of EIAs in providing accurate and rapid diagnostic information becomes increasingly vital. This demand is further driven by expanding healthcare access in developing economies and the increasing availability of diagnostic testing in various healthcare settings.
- Furthermore, technological advancements in EIA assays, such as improved sensitivity, specificity, and ease of use, are making them an increasingly preferred choice for a wide range of diagnostic applications. The development of multiplex assays, which can simultaneously detect multiple analytes, and the integration of EIAs with automated platforms, are key factors propelling their adoption.
- The convenience, cost-effectiveness, and versatility of EIAs in detecting a wide range of biomarkers and pathogens are key factors driving their adoption in both clinical and research settings. The increasing availability of user-friendly EIA kits and the expanding applications of EIAs in point-of-care testing further contribute to market growth.
Restraint/Challenge
“Stringent and varying regulatory requirements”
- Complex Regulatory Landscape and High Development Costs: The Enzyme Immunoassay (EIA) Reagents and Devices market is subject to stringent regulatory requirements and quality control standards, which can vary across different regions and countries.
- For instance, obtaining regulatory approvals for new EIA-based diagnostic tests can be a complex and time-consuming process, requiring extensive clinical trials and validation studies. Similarly, compliance with quality management systems, such as ISO 13485, adds to the operational burden and costs for manufacturers.
- Addressing these regulatory challenges requires manufacturers to invest heavily in research and development, adhere to rigorous manufacturing practices, and maintain robust quality control systems. The high costs associated with developing and commercializing new EIA products, coupled with the need for ongoing compliance, can be a significant barrier to entry for smaller companies and may limit innovation in the market.
- Moreover, the increasing complexity of EIA assays, such as multiplex assays and those targeting emerging biomarkers, often necessitates advanced technologies and expertise, further driving up development costs.
- Overcoming these challenges through streamlined regulatory pathways, greater harmonization of global standards, and the development of cost-effective manufacturing and quality control solutions will be vital for fostering innovation and ensuring the widespread availability of high-quality EIA products
Enzyme Immunoassay (EIA) Reagents and Devices Market Scope
The market is segmented on the basis of technology, product type, application and end users.
- By Technology
On the basis of technology, the enzyme immunoassay (EIA) reagents and devices market is segmented into enzyme immunoassays, fluorescent immunoassays, chemiluminescence, immunoassays, radioimmunoassay and others. The Enzyme Immunoassays segment dominates the largest market revenue share of 43.2% in 2025, driven by its versatility, wide range of applications, and established use in various diagnostic and research settings. Laboratories often prioritize ELISA Enzyme Immunoassay (EIA) Reagents and Devices for their reliability, cost-effectiveness, and ability to be adapted to detect a wide variety of analytes. The market also sees strong demand for ELISA due to its compatibility with automation and high-throughput screening, making it a standard tool in clinical diagnostics and research.
The Chemiluminescence Immunoassay segment is anticipated to witness the fastest growth rate of 21.7% from 2025 to 2032 fueled by increasing adoption in high-sensitivity applications such as infectious disease testing, and immunochemistry. Chemiluminescence Immunoassays offer enhanced sensitivity and a wide dynamic range, making them suitable for detecting low-abundance analytes. Their integration with automated platforms and ability to provide rapid results also contribute to their growing popularity in modern clinical laboratories.
- By Product type
On the basis of product type, the enzyme immunoassay (EIA) reagents and devices market is segmented into analysers and reagents. The Reagents held the largest market revenue share in 2025 of, driven by the recurring need for consumables in EIA testing. EIA tests require a consistent supply of reagents, including antibodies, enzymes, and other solutions, making reagents a fundamental and continuously in-demand product segment. The increasing volume of EIA tests performed globally, due to the rising prevalence of diseases and expanding healthcare access, fuels the reagents market.
The Analyzers segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing automation of EIA workflows. Automated EIA analyzers offer high-throughput processing, reduced turnaround times, and improved accuracy, which are critical for modern laboratories. The demand for analyzers is also growing due to the increasing adoption of advanced technologies like robotics and software integration in laboratories.
- By Application
On the basis of application, the enzyme immunoassay (EIA) reagents and devices market is segmented into oncology, infectious diseases, cardiology, bone and mineral, endocrinology, autoimmunity, toxicology, haematology, neonatal screening, and others. The Infectious disease held the largest market revenue share in 2025 driven by the critical role of EIAs in the diagnosis and management of a wide range of infectious diseases, including HIV, hepatitis, and emerging pathogens. EIAs are extensively used due to their cost-effectiveness, high throughput, and suitability for screening large populations. The increasing prevalence of infectious diseases worldwide and the growing need for accurate and rapid diagnostic tools contribute to the dominance of this segment.
The oncology testing is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing use of EIAs in cancer screening, diagnosis, and monitoring. EIAs are used to detect tumor markers and other biomarkers associated with various cancers, enabling early detection and personalized treatment strategies. The rising incidence of cancer globally and the development of novel cancer biomarkers are fueling the growth of this segment.
- By End users
On the basis of end users, the enzyme immunoassay (EIA) reagents and devices market is segmented into hospitals, laboratories, academics, pharmaceutical industries and others. The Hospitals segment accounted for the largest market revenue share in 2024, driven by the high volume of diagnostic testing conducted in these facilities. Hospitals utilize EIA reagents and devices extensively for routine blood tests, disease diagnosis, and patient monitoring. The increasing number of hospital admissions, advancements in medical technology, and the growing focus on improving patient care contribute to the dominance of this segment.
The Pharmaceutical Industries segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing use of EIAs in drug discovery, development, and quality control. EIAs are employed to analyze drug efficacy, detect impurities, and ensure product safety. The expanding pharmaceutical industry, the rise of biopharmaceuticals, and the growing emphasis on research and development are fueling the growth of this segment.
Enzyme Immunoassay (EIA) Reagents and Devices Market Regional Analysis
- North America dominates the Enzyme Immunoassay (EIA) Reagents and Devices market with the largest revenue share of 40.01% in 2024, driven by a growing demand for advanced diagnostics and the strong presence of major pharmaceutical and biotechnology companies
- The region's well-established healthcare infrastructure, high healthcare expenditure, and widespread adoption of advanced medical technologies contribute to its market leadership.
U.S. Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The U.S. Enzyme Immunoassay (EIA) Reagents and Devices market captured the largest revenue share of 81% within North America in 2025, fueled by the increasing prevalence of chronic diseases, rising geriatric population, and the growing demand for early and accurate diagnostic testing. The presence of numerous leading research institutions and pharmaceutical companies in the U.S. further drives market growth through continuous innovation and the development of novel EIA-based assays. Moreover, favorable reimbursement policies and a strong focus on personalized medicine contribute to the market's expansion.
Europe Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The European Enzyme Immunoassay (EIA) Reagents and Devices market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by the increasing adoption of in-vitro diagnostics, rising healthcare expenditure, and growing awareness of preventive healthcare. The market is also driven by the increasing prevalence of chronic diseases and the expanding geriatric population across European countries. Additionally, stringent regulatory standards and the presence of well-established healthcare systems contribute to the market's growth.
U.K. Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The U.K. Enzyme Immunoassay (EIA) Reagents and Devices market is anticipated to grow at a noteworthy during the forecast period, driven by the increasing focus on early disease detection, the rising prevalence of chronic diseases, and the expansion of diagnostic testing facilities. The UK's strong healthcare system, coupled with government initiatives to improve diagnostic services, is expected to continue to stimulate market growth. Furthermore, the growing adoption of point-of-care testing and the increasing demand for cost-effective diagnostic solutions are fueling market growth.
Germany Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The German Enzyme Immunoassay (EIA) Reagents and Devices market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of advanced diagnostics and the demand for high-quality healthcare solutions. Germany's well-developed healthcare infrastructure, combined with its emphasis on research and development, promotes the adoption of innovative EIA-based technologies, particularly in clinical diagnostics and research settings. The increasing focus on personalized medicine and the growing geriatric population are also contributing to the market's expansion.
Asia-Pacific Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The Asia-Pacific Enzyme Immunoassay (EIA) Reagents and Devices market is poised to grow at the fastest CAGR of over 24% in 2025 driven by increasing prevalence of infectious diseases, expanding healthcare access, and rising healthcare expenditure in countries such as China, Japan, and India. The region's growing emphasis on improving healthcare infrastructure, coupled with rising disposable incomes, is driving the adoption of EIA reagents and devices. Furthermore, the increasing awareness of early disease detection and the growing demand for cost-effective diagnostic solutions are fueling market growth in this region.
Japan Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The Japan Enzyme Immunoassay (EIA) Reagents and Devices market is gaining momentum due to the country’s focus on technological advancements in healthcare, a rapidly aging population, and increasing demand for high-quality diagnostic testing. The Japanese market places a significant emphasis on precision and reliability in diagnostics, and the adoption of EIA reagents and devices is driven by the increasing need for accurate and efficient disease management. Moreover, the growing geriatric population and the increasing prevalence of chronic diseases are driving the demand for advanced diagnostic solutions like EIAs.
China Enzyme Immunoassay (EIA) Reagents and Devices Market Insight
The China Enzyme Immunoassay (EIA) Reagents and Devices market accounted for the largest market revenue share in Asia Pacific in 2025 attributed to the country's expanding healthcare infrastructure, increasing government support for the healthcare sector, and rising awareness of advanced diagnostic technologies. China represents one of the largest and fastest-growing markets for in-vitro diagnostics, with EIAs being increasingly utilized in hospitals, clinical laboratories, and research institutions. The increasing prevalence of infectious diseases, the growing middle class, and rising healthcare expenditure are key factors propelling the market in China.
Enzyme Immunoassay (EIA) Reagents and Devices Market Share
The Enzyme Immunoassay (EIA) Reagents and Devices industry is primarily led by well-established companies, including:
- Abbott Laboratories (U.S.)
- Roche Diagnostics (Switzerland)
- Siemens Healthineers (Germany)
- Thermo Fisher Scientific (U.S.)
- Bio-Rad Laboratories (U.S.)
- Danaher Corporation (U.S.)
- DiaSorin S.p.A. (Italy)
- Ortho Clinical Diagnostics (U.S.)
- Merck KGaA (Germany)
- Agilent Technologies (U.S.)
- Beckman Coulter, Inc. (U.S.)
- Fujirebio Holdings Inc. (Japan)
- ZEUS Scientific (U.S.)
- ELISA Technologies, Inc. (U.S.)
- Creative Diagnostics (U.S.)
- RayBiotech, Inc. (U.S.)
- R&D Systems, Inc. (U.S.)
- BD (Becton, Dickinson and Company) (U.S.)
- bioMérieux S.A. (France)
- Diagnostic Automation / Cortez Diagnostics, Inc. (U.S)
Latest Developments in Global Enzyme Immunoassay (EIA) Reagents and Devices Market
- In December 2023, QuidelOrtho Corporation received FDA clearance for its VITROS® Anti-SARS-CoV-2 IgG Quantitative Test. This test measures the amount of IgG antibodies to the SARS-CoV-2 virus, providing valuable information for assessing the immune response to infection or vaccination. This clearance enhances QuidelOrtho's portfolio of diagnostic solutions for COVID-19 and highlights the ongoing development of advanced EIA-based tests to address the pandemic.
- In November 2023, DiaSorin launched the LIAISON® XL Anti-SARS-CoV-2 TrimericS IgG assay. This assay is designed to detect IgG antibodies against the trimeric spike protein of SARS-CoV-2, which may offer improved sensitivity and specificity compared to assays targeting other viral antigens. The development of this assay demonstrates DiaSorin's commitment to providing innovative serological tools for COVID-19 research and clinical management.
- In October 2023, Siemens Healthineers introduced the Atellica IM Analyzer, a high-throughput immunoassay system designed to meet the growing demands of clinical laboratories. This analyzer offers a wide range of immunoassay tests, including those based on EIA principles, with enhanced automation and efficiency. The launch of the Atellica® IM Analyzer reflects the industry trend towards increased automation and digitalization in EIA testing.
- In September 2023, Thermo Fisher Scientific expanded its portfolio of Thermo Scientific ELISA kits with the addition of several new assays for detecting biomarkers in various disease areas, including autoimmune disorders, cancer, and infectious diseases. These new ELISA kits are designed to provide researchers with reliable and easy-to-use tools for their studies. This expansion highlights the continued importance of ELISA assays in research and development.
- In August 2023, Bio-Rad Laboratories received CE-IVD mark for its Platelia™ SARS-CoV-2 Total Ab assay. This assay detects total antibodies (IgG, IgM, and IgA) to SARS-CoV-2 and can be used to assess past or present infection. The CE-IVD mark allows for the use of this assay in European markets and further expands Bio-Rad's offerings for COVID-19 diagnostics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.