Market Analysis and Insights Global Craniomaxillofacial Implants Market
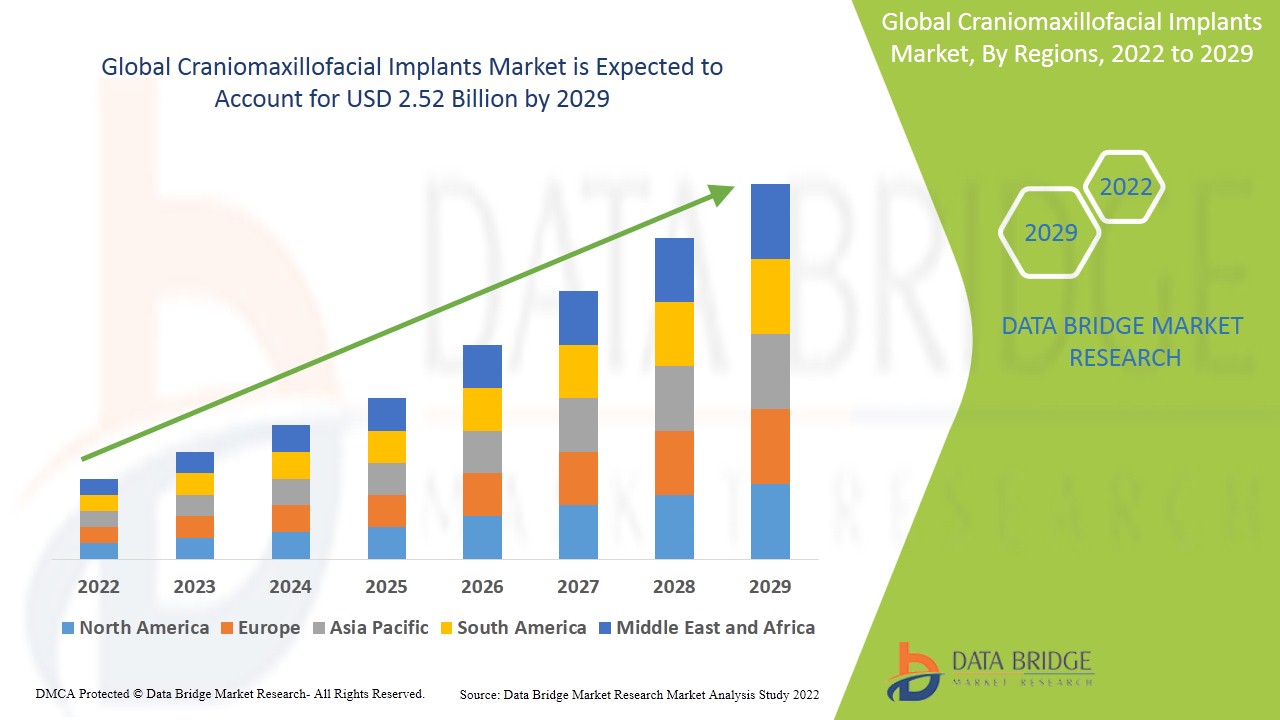
Data Bridge Market Research analyses that the craniomaxillofacial implants market to account USD 2.52 billion by 2029 and growing at a CAGR of 7.40% in the forecast period of 2022-2029.
Reconstructive dental and craniomaxillofacial implant surgery encompasses the use of implants to rehabilitate and restore kind and performance to the toothless or part toothless jaws and therefore the craniomaxillofacial skeleton of patients mounted and removable prostheses.
The growing demand of minimally invasive reconstruction surgeries is the major factor accelerating the growth of the craniomaxillofacial implants market. Furthermore improved technological advanced products and rising numbers of trauma cases are also expected to drive the growth of the craniomaxillofacial implants market. However, high cost of craniomaxillofacial surgeries restrains the craniomaxillofacial implants market, whereas, risk associated with the implant malfunction will challenge market growth.
In addition, growing prevalence of numerous players will create ample opportunities for the craniomaxillofacial implants market.
This craniomaxillofacial implants market report provides details of new recent developments, trade regulations, import export analysis, production analysis, value chain optimization, market share, impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on craniomaxillofacial implants market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Craniomaxillofacial Implants Market Scope and Market Size
The craniomaxillofacial implants market is segmented on the basis of type, material of construction, material of application site and property. The growth amongst these segments will help you analyse meagre growth segments in the industries, and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
- Based on type, the craniomaxillofacial implants market is segmented into mid face, plates, screws, mandibular orthognathic implants, neuro, mesh, bone graft, dural repair. Mid Face Implants is further segmented into screws, plates. Mandibular Orthognathic Implants is further segmented into screws, plates. Cranial or Neuro Implants is further segmented into screws, plates, contourable mesh. Dural Repair Products is further segmented into dural Substitutes, dural sealants.
- Based on material of construction the craniomaxillofacial implants market is segmented into calcium phosphate ceramics, titanium, alloys and other metals, polymers Or biomaterials.
- Based on material of application site the craniomaxillofacial implants market is segmented into internal fixators andexternal fixators.
- Based on property the craniomaxillofacial implants market is segmented into resorbable fixators andnon-resorbable fixators.
Craniomaxillofacial Implants Market Country Level Analysis
The craniomaxillofacial implants market is analysed and market size insights and trends are provided by country, type, material of construction, material of application site and property as referenced above.
The countries covered in the craniomaxillofacial implants market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the craniomaxillofacial implants market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to increasing healthcare expenditure and growth of sports injuries, and injuries related to cranial and facial bones in this region. Asia-Pacific on the other hand is projected to exhibit the highest growth rate during the forecast period due to increasing road accidents, growing sports injuries, and increasing aging population.
The country section of the craniomaxillofacial implants market report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as consumption volumes, production sites and volumes, import export analysis, price trend analysis, cost of raw materials, down-stream and upstream value chain analysis are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The craniomaxillofacial implants market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for craniomaxillofacial implants market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the craniomaxillofacial implants market. The data is available for historic period 2010 to 2029.
Competitive Landscape and Craniomaxillofacial Implants Market Share Analysis
The craniomaxillofacial implants market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to craniomaxillofacial implants market.
Some of the major players operating in the craniomaxillofacial implants market report are Zimmer Biomet, Stryker, Medartis AG, KLS Martin Group, Depuy Synthes, Osteomed, Integra Life Sciences Corporation, Medtronic, Calavera Surgical Design, B. Braun Melsungen AG, General Implants GmbH, Rebstock Instruments GmbH, BIOPORE Surgical Implants, Poriferous, LLC, Osteotec, Johnson & Johnson Services, Inc., Anatomics Pty. Ltd. among other
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 APPLICATION POSITIONING GRID
2.2.5 COMPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 EPIDEMIOLOGY MODELIING
2.2.8 TOP TO BOTTOM ANALYSIS
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY ANATOMY
16.1 OVERVIEW
16.2 THORACIC
16.3 NEURO
16.4 ORBITAL
16.5 MANDIBLE
16.6 OTHERS
17 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PRODUCT TYPE
17.1 OVERVIEW
17.2 MID-FACE IMPLANTS
17.2.1 PLATES
17.2.2 SCREWS
17.3 CRANIAL/NEURO IMPLANTS
17.3.1 PLATES
17.3.2 CONTOURABLE MESHES
17.3.3 SCREWS
17.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
17.4.1 PLATES
17.4.2 SCREWS
17.5 BONE GRAFT SUBSTITUTE
17.5.1 SYNTHETIC
17.5.2 NATURAL
17.6 DURAL REPAIR PRODUCT
17.6.1 DURAL SUBSTITUTES
17.6.2 DURAL SEALANTS
17.7 TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
17.8 CRANIAL FLAP FIXATION SYSTEMS
17.9 THORACIC FIXATION SYSTEMS
17.1 DISTRACTION SYSTEM
17.11 OTHERS
18 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL
18.1 OVERVIEW
18.2 METALS
18.2.1 STAINLESS STEEL
18.2.2 TITANIUM AND ALLOYS
18.2.3 POLYETHERETHERKETONE (PEEK)
18.2.4 SILICON NITRIDE
18.2.5 OTHERS
18.3 BONE GRAFT SUBSTITUTE
18.4 POLYMERS/BIOMATERIAL
18.5 CALCIUM PHOSPHATE CERAMICS
18.6 OTHERS
19 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE
19.1 OVERVIEW
19.2 INTERNAL FIXATORS
19.2.1 MID-FACE IMPLANTS
19.2.2 CRANIAL/NEURO IMPLANTS
19.2.3 MANDIBULAR ORTHOGNATHIC IMPLANTS
19.2.4 BONE GRAFT SUBSTITUTE
19.2.5 DURAL REPAIR PRODUCT
19.2.6 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
19.2.7 CRANIAL FLAP FIXATION SYSTEMS
19.2.8 THORACIC FIXATION SYSTEMS
19.2.9 DISTRACTION SYSTEM
19.2.10 OTHERS
19.3 EXTERNAL FIXATORS
19.3.1 PATIENT SPECIFIC IMPLANTS (PSI)
19.3.2 MID-FACE IMPLANTS
19.3.3 CRANIAL/NEURO IMPLANTS
19.3.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
19.3.5 BONE GRAFT SUBSTITUTE
19.3.6 DURAL REPAIR PRODUCT
19.3.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
19.3.8 CRANIAL FLAP FIXATION SYSTEMS
19.3.9 THORACIC FIXATION SYSTEMS
19.3.10 DISTRACTION SYSTEM
19.3.11 OTHERS
20 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE
20.1 OVERVIEW
20.2 ORTHOGNATHIC SURGERIES
20.2.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.2.2 MID-FACE IMPLANTS
20.2.3 CRANIAL/NEURO IMPLANTS
20.2.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.2.5 BONE GRAFT SUBSTITUTE
20.2.6 DURAL REPAIR PRODUCT
20.2.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.2.8 CRANIAL FLAP FIXATION SYSTEMS
20.2.9 THORACIC FIXATION SYSTEMS
20.2.10 DISTRACTION SYSTEM
20.2.11 OTHERS
20.3 PLASTIC SURGERIES
20.3.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.3.2 MID-FACE IMPLANTS
20.3.3 CRANIAL/NEURO IMPLANTS
20.3.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.3.5 BONE GRAFT SUBSTITUTE
20.3.6 DURAL REPAIR PRODUCT
20.3.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.3.8 CRANIAL FLAP FIXATION SYSTEMS
20.3.9 THORACIC FIXATION SYSTEMS
20.3.10 DISTRACTION SYSTEM
20.3.11 OTHERS
20.4 TRAUMA SURGERIES
20.4.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.4.2 MID-FACE IMPLANTS
20.4.3 CRANIAL/NEURO IMPLANTS
20.4.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.4.5 BONE GRAFT SUBSTITUTE
20.4.6 DURAL REPAIR PRODUCT
20.4.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.4.8 CRANIAL FLAP FIXATION SYSTEMS
20.4.9 THORACIC FIXATION SYSTEMS
20.4.10 DISTRACTION SYSTEM
20.4.11 OTHERS
20.5 RECONSTRUCTIVE SURGERY
20.5.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.5.2 MID-FACE IMPLANTS
20.5.3 CRANIAL/NEURO IMPLANTS
20.5.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.5.5 BONE GRAFT SUBSTITUTE
20.5.6 DURAL REPAIR PRODUCT
20.5.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.5.8 CRANIAL FLAP FIXATION SYSTEMS
20.5.9 THORACIC FIXATION SYSTEMS
20.5.10 DISTRACTION SYSTEM
20.5.11 OTHERS
20.6 ENT SURGERIES
20.6.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.6.2 MID-FACE IMPLANTS
20.6.3 CRANIAL/NEURO IMPLANTS
20.6.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.6.5 BONE GRAFT SUBSTITUTE
20.6.6 DURAL REPAIR PRODUCT
20.6.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.6.8 CRANIAL FLAP FIXATION SYSTEMS
20.6.9 THORACIC FIXATION SYSTEMS
20.6.10 DISTRACTION SYSTEM
20.6.11 OTHERS
20.7 DENTAL SURGERIES
20.7.1 PATIENT SPECIFIC IMPLANTS (PSI)
20.7.2 MID-FACE IMPLANTS
20.7.3 CRANIAL/NEURO IMPLANTS
20.7.4 MANDIBULAR ORTHOGNATHIC IMPLANTS
20.7.5 BONE GRAFT SUBSTITUTE
20.7.6 DURAL REPAIR PRODUCT
20.7.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
20.7.8 CRANIAL FLAP FIXATION SYSTEMS
20.7.9 THORACIC FIXATION SYSTEMS
20.7.10 DISTRACTION SYSTEM
20.7.11 OTHERS
20.8 OTHERS
21 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY
21.1 OVERVIEW
21.2 RESORBABLE FIXATORS
21.3 NON-RESORBABLE FIXATORS
22 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE OF IMPLANT
22.1 OVERVIEW
22.2 CUSTOM MADE
22.3 READY MADE
23 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER
23.1 OVERVIEW
23.2 HOSPITALS
23.2.1 PUBLIC
23.2.2 PRIVATE
23.3 SPECILATY CLINICS
23.3.1 PUBLIC
23.3.2 PRIVATE
23.4 TRAUMA CENTERS
23.5 AMBULATORY SURGICAL CENTERS (ASCS)
23.6 OTHERS
24 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL
24.1 OVERVIEW
24.2 DIRECT TENDER
24.3 RETAIL SALES
24.3.1 ONLINE
24.3.2 OFFLINE
24.4 OTHERS
25 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY GEOGRAPHY
GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 NORTH AMERICA
25.1.1 U.S.
25.1.2 CANADA
25.1.3 MEXICO
25.2 EUROPE
25.2.1 GERMANY
25.2.2 U.K.
25.2.3 ITALY
25.2.4 FRANCE
25.2.5 SPAIN
25.2.6 RUSSIA
25.2.7 SWITZERLAND
25.2.8 TURKEY
25.2.9 BELGIUM
25.2.10 NETHERLANDS
25.2.11 DENMARK
25.2.12 SWEDEN
25.2.13 POLAND
25.2.14 NORWAY
25.2.15 FINLAND
25.2.16 REST OF EUROPE
25.3 ASIA-PACIFIC
25.3.1 JAPAN
25.3.2 CHINA
25.3.3 SOUTH KOREA
25.3.4 INDIA
25.3.5 SINGAPORE
25.3.6 THAILAND
25.3.7 INDONESIA
25.3.8 MALAYSIA
25.3.9 PHILIPPINES
25.3.10 AUSTRALIA
25.3.11 NEW ZEALAND
25.3.12 VIETNAM
25.3.13 TAIWAN
25.3.14 REST OF ASIA-PACIFIC
25.4 SOUTH AMERICA
25.4.1 BRAZIL
25.4.2 ARGENTINA
25.4.3 REST OF SOUTH AMERICA
25.5 MIDDLE EAST AND AFRICA
25.5.1 SOUTH AFRICA
25.5.2 EGYPT
25.5.3 BAHRAIN
25.5.4 UNITED ARAB EMIRATES
25.5.5 KUWAIT
25.5.6 OMAN
25.5.7 QATAR
25.5.8 SAUDI ARABIA
25.5.9 REST OF MEA
25.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
26 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, COMPANY LANDSCAPE
26.1 COMPANY SHARE ANALYSIS: GLOBAL
26.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
26.3 COMPANY SHARE ANALYSIS: EUROPE
26.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
26.5 MERGERS & ACQUISITIONS
26.6 NEW PRODUCT DEVELOPMENT & APPROVALS
26.7 EXPANSIONS
26.8 REGULATORY CHANGES
26.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
27 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, SWOT AND DBMR ANALYSIS
28 GLOBAL CRANIOMAXILLOFACIAL IMPLANTS MARKET, COMPANY PROFILE
28.1 STRYKER
28.1.1 COMPANY OVERVIEW
28.1.2 REVENUE ANALYSIS
28.1.3 GEOGRAPHIC PRESENCE
28.1.4 PRODUCT PORTFOLIO
28.1.5 RECENT DEVELOPMENTS
28.2 NARANG MEDICAL LIMITED
28.2.1 COMPANY OVERVIEW
28.2.2 REVENUE ANALYSIS
28.2.3 GEOGRAPHIC PRESENCE
28.2.4 PRODUCT PORTFOLIO
28.2.5 RECENT DEVELOPMENTS
28.3 ZIMMER BIOMET
28.3.1 COMPANY OVERVIEW
28.3.2 REVENUE ANALYSIS
28.3.3 GEOGRAPHIC PRESENCE
28.3.4 PRODUCT PORTFOLIO
28.3.5 RECENT DEVELOPMENTS
28.4 MEDARTIS AG
28.4.1 COMPANY OVERVIEW
28.4.2 REVENUE ANALYSIS
28.4.3 GEOGRAPHIC PRESENCE
28.4.4 PRODUCT PORTFOLIO
28.4.5 RECENT DEVELOPMENTS
28.5 KLS MARTIN GROUP
28.5.1 COMPANY OVERVIEW
28.5.2 REVENUE ANALYSIS
28.5.3 GEOGRAPHIC PRESENCE
28.5.4 PRODUCT PORTFOLIO
28.5.5 RECENT DEVELOPMENTS
28.6 DEPUY SYNTHES (JOHNSON & JOHNSON SERVICES, INC.)
28.6.1 COMPANY OVERVIEW
28.6.2 REVENUE ANALYSIS
28.6.3 GEOGRAPHIC PRESENCE
28.6.4 PRODUCT PORTFOLIO
28.6.5 RECENT DEVELOPMENTS
28.7 ACUMED LLC, A COLSON MEDICAL
28.7.1 COMPANY OVERVIEW
28.7.2 REVENUE ANALYSIS
28.7.3 GEOGRAPHIC PRESENCE
28.7.4 PRODUCT PORTFOLIO
28.7.5 RECENT DEVELOPMENTS
28.8 INTEGRA LIFESCIENCES CORPORATION
28.8.1 COMPANY OVERVIEW
28.8.2 REVENUE ANALYSIS
28.8.3 GEOGRAPHIC PRESENCE
28.8.4 PRODUCT PORTFOLIO
28.8.5 RECENT DEVELOPMENTS
28.9 MEDTRONIC
28.9.1 COMPANY OVERVIEW
28.9.2 REVENUE ANALYSIS
28.9.3 GEOGRAPHIC PRESENCE
28.9.4 PRODUCT PORTFOLIO
28.9.5 RECENT DEVELOPMENTS
28.1 B. BRAUN MELSUNGEN AG
28.10.1 COMPANY OVERVIEW
28.10.2 REVENUE ANALYSIS
28.10.3 GEOGRAPHIC PRESENCE
28.10.4 PRODUCT PORTFOLIO
28.10.5 RECENT DEVELOPMENTS
28.11 GENERAL IMPLANTS GMBH GERMANY
28.11.1 COMPANY OVERVIEW
28.11.2 REVENUE ANALYSIS
28.11.3 GEOGRAPHIC PRESENCE
28.11.4 PRODUCT PORTFOLIO
28.11.5 RECENT DEVELOPMENTS
28.12 REBSTOCK INSTRUMENTS GMBH
28.12.1 COMPANY OVERVIEW
28.12.2 REVENUE ANALYSIS
28.12.3 GEOGRAPHIC PRESENCE
28.12.4 PRODUCT PORTFOLIO
28.12.5 RECENT DEVELOPMENTS
28.13 PORIFEROUS LLC
28.13.1 COMPANY OVERVIEW
28.13.2 REVENUE ANALYSIS
28.13.3 GEOGRAPHIC PRESENCE
28.13.4 PRODUCT PORTFOLIO
28.13.5 RECENT DEVELOPMENTS
28.14 ORTHOFIX MEDICAL INC
28.14.1 COMPANY OVERVIEW
28.14.2 REVENUE ANALYSIS
28.14.3 GEOGRAPHIC PRESENCE
28.14.4 PRODUCT PORTFOLIO
28.14.5 RECENT DEVELOPMENTS
28.15 TITAMED
28.15.1 COMPANY OVERVIEW
28.15.2 REVENUE ANALYSIS
28.15.3 GEOGRAPHIC PRESENCE
28.15.4 PRODUCT PORTFOLIO
28.15.5 RECENT DEVELOPMENTS
28.16 DELPHOS IMPLANTS SA
28.16.1 COMPANY OVERVIEW
28.16.2 REVENUE ANALYSIS
28.16.3 GEOGRAPHIC PRESENCE
28.16.4 PRODUCT PORTFOLIO
28.16.5 RECENT DEVELOPMENTS
28.17 ORTHO BALTIC IMPLANTS
28.17.1 COMPANY OVERVIEW
28.17.2 REVENUE ANALYSIS
28.17.3 GEOGRAPHIC PRESENCE
28.17.4 PRODUCT PORTFOLIO
28.17.5 RECENT DEVELOPMENTS
28.18 JEIL MEDICAL CORPORATION
28.18.1 COMPANY OVERVIEW
28.18.2 REVENUE ANALYSIS
28.18.3 GEOGRAPHIC PRESENCE
28.18.4 PRODUCT PORTFOLIO
28.18.5 RECENT DEVELOPMENTS
28.19 INION OY
28.19.1 COMPANY OVERVIEW
28.19.2 REVENUE ANALYSIS
28.19.3 GEOGRAPHIC PRESENCE
28.19.4 PRODUCT PORTFOLIO
28.19.5 RECENT DEVELOPMENTS
28.2 MATERIALISE
28.20.1 COMPANY OVERVIEW
28.20.2 REVENUE ANALYSIS
28.20.3 GEOGRAPHIC PRESENCE
28.20.4 PRODUCT PORTFOLIO
28.20.5 RECENT DEVELOPMENTS
28.21 NARANG MEDICAL LIMITED.
28.21.1 COMPANY OVERVIEW
28.21.2 REVENUE ANALYSIS
28.21.3 GEOGRAPHIC PRESENCE
28.21.4 PRODUCT PORTFOLIO
28.21.5 RECENT DEVELOPMENTS
28.22 PRECIMA TECHNOLOGIES
28.22.1 COMPANY OVERVIEW
28.22.2 REVENUE ANALYSIS
28.22.3 GEOGRAPHIC PRESENCE
28.22.4 PRODUCT PORTFOLIO
28.22.5 RECENT DEVELOPMENTS
28.23 GLOBAL ORTHOSYS
28.23.1 COMPANY OVERVIEW
28.23.2 REVENUE ANALYSIS
28.23.3 GEOGRAPHIC PRESENCE
28.23.4 PRODUCT PORTFOLIO
28.23.5 RECENT DEVELOPMENTS
28.24 OSTEOPHOENIX
28.24.1 COMPANY OVERVIEW
28.24.2 REVENUE ANALYSIS
28.24.3 GEOGRAPHIC PRESENCE
28.24.4 PRODUCT PORTFOLIO
28.24.5 RECENT DEVELOPMENTS
28.25 POLY-MED INCORPORATED
28.25.1 COMPANY OVERVIEW
28.25.2 REVENUE ANALYSIS
28.25.3 GEOGRAPHIC PRESENCE
28.25.4 PRODUCT PORTFOLIO
28.25.5 RECENT DEVELOPMENTS
28.26 GPC MEDICAL LTD.
28.26.1 COMPANY OVERVIEW
28.26.2 REVENUE ANALYSIS
28.26.3 GEOGRAPHIC PRESENCE
28.26.4 PRODUCT PORTFOLIO
28.26.5 RECENT DEVELOPMENTS
28.27 GREENS SURGICALS PVT. LTD.
28.27.1 COMPANY OVERVIEW
28.27.2 REVENUE ANALYSIS
28.27.3 GEOGRAPHIC PRESENCE
28.27.4 PRODUCT PORTFOLIO
28.27.5 RECENT DEVELOPMENTS
28.28 CHANGZHOU MEDITECH TECHNOLOGY CO., LTD
28.28.1 COMPANY OVERVIEW
28.28.2 REVENUE ANALYSIS
28.28.3 GEOGRAPHIC PRESENCE
28.28.4 PRODUCT PORTFOLIO
28.28.5 RECENT DEVELOPMENTS
28.29 DOUBLE MEDICAL TECHNOLOGY INC.
28.29.1 COMPANY OVERVIEW
28.29.2 REVENUE ANALYSIS
28.29.3 GEOGRAPHIC PRESENCE
28.29.4 PRODUCT PORTFOLIO
28.29.5 RECENT DEVELOPMENTS
28.3 ACUMED LLC, A COLSON MEDICAL
28.30.1 COMPANY OVERVIEW
28.30.2 REVENUE ANALYSIS
28.30.3 GEOGRAPHIC PRESENCE
28.30.4 PRODUCT PORTFOLIO
28.30.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
29 RELATED REPORTS
30 CONCLUSION
31 QUESTIONNAIRE
32 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.