Europe Electrophysiology Market
Market Size in USD Billion
CAGR :
% 
 USD
2.83 Billion
USD
5.04 Billion
2025
2033
USD
2.83 Billion
USD
5.04 Billion
2025
2033
| 2026 –2033 | |
| USD 2.83 Billion | |
| USD 5.04 Billion | |
|
|
|
|
Europe Electrophysiology Market Size
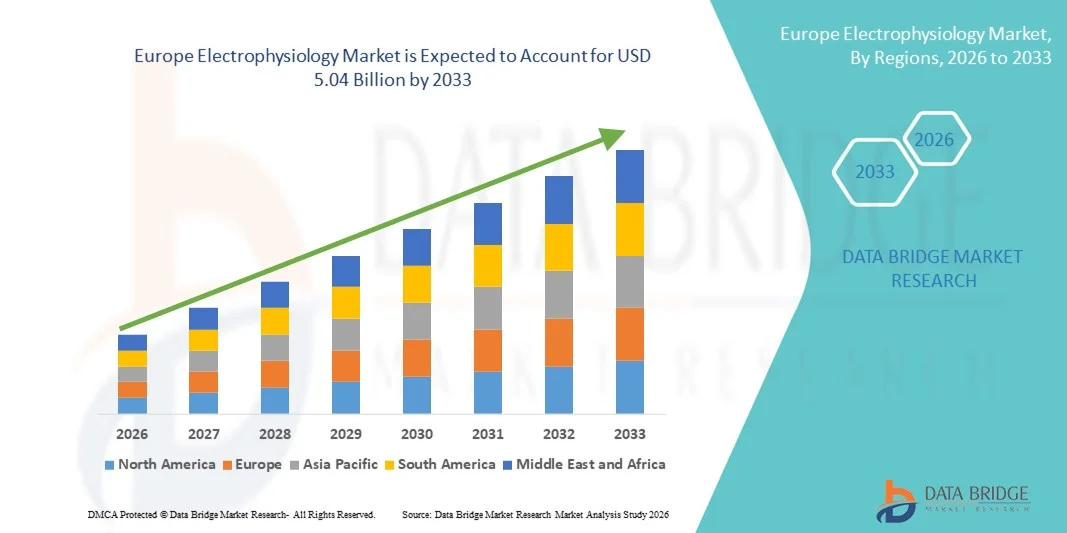
- The Europe electrophysiology market size was valued at USD 2.83 billion in 2025 and is expected to reach USD 5.04 billion by 2033, at a CAGR of 7.5% during the forecast period
- The market growth is largely fueled by advancements in electrophysiology technologies, including innovative ablation catheters, mapping systems, and diagnostic tools, alongside rising adoption of minimally invasive cardiac procedures and enhanced healthcare infrastructure in major European countries
- Furthermore, growing investments in electrophysiology laboratories, the ageing population with higher cardiovascular disease incidence, and supportive reimbursement and public awareness initiatives are increasing the uptake of electrophysiology solutions in both clinical and hospital settings establishing EP devices as essential tools for arrhythmia management and cardiac care across Europe
Europe Electrophysiology Market Analysis
- Electrophysiology devices, including diagnostic and therapeutic tools for cardiac arrhythmias, are increasingly vital components of modern cardiovascular care in both hospital and clinical settings due to their precision, minimally invasive procedures, and integration with advanced imaging and mapping systems
- The escalating demand for electrophysiology solutions is primarily fueled by rising prevalence of arrhythmias and other cardiovascular disorders, growing awareness of advanced treatment options, and increasing adoption of minimally invasive catheter-based procedures
- Germany dominated the Europe electrophysiology market with the largest revenue share of 38.5% in 2025, characterized by well-established healthcare infrastructure, high adoption of advanced cardiac technologies, and strong presence of leading EP device manufacturers, with substantial growth driven by investments in EP labs and innovations in mapping and ablation systems
- Poland is expected to be the fastest growing country in the Europe electrophysiology market during the forecast period due to increasing healthcare expenditure, expansion of cardiac care facilities, and rising patient awareness regarding arrhythmia management
- Ablation catheters segment dominated the electrophysiology market with a market share of 45.7% in 2025, driven by their proven efficacy in treating arrhythmias, technological advancements in catheter design, and widespread adoption across hospitals and specialty cardiac centers
Report Scope and Europe Electrophysiology Market Segmentation
|
Attributes |
Europe Electrophysiology Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Europe Electrophysiology Market Trends
Advancements Through AI-Enabled Mapping and Remote Monitoring
- A significant and accelerating trend in the Europe electrophysiology market is the integration of artificial intelligence (AI) into cardiac mapping systems and remote monitoring solutions, enhancing procedural precision and patient management in both hospital and outpatient settings
- For instance, the CARTO 3 System by Biosense Webster integrates AI-driven mapping with real-time data analysis, allowing electrophysiologists to navigate complex arrhythmias more efficiently and improve procedural outcomes
- AI integration enables features such as predicting arrhythmia recurrence, optimizing ablation strategies, and providing intelligent alerts for patient follow-ups. For instance, the Ensite X EP System uses AI algorithms to improve electroanatomical mapping accuracy and suggest ablation targets based on patient-specific data
- The integration of EP devices with remote patient monitoring platforms allows clinicians to track cardiac activity and device performance from a centralized interface, facilitating timely interventions and reducing hospital visits
- This trend toward intelligent, data-driven, and interconnected EP solutions is reshaping procedural standards and clinical expectations. Consequently, companies such as Abbott are developing AI-enabled mapping and ablation systems with real-time predictive analytics to enhance patient safety and procedural efficiency
- The adoption of AI-powered and remotely monitored EP solutions is growing rapidly across both leading and emerging European countries, as healthcare providers increasingly prioritize procedural precision, patient convenience, and improved outcomes
- Collaborations between EP device manufacturers and digital health platforms are accelerating, driving innovation in predictive analytics, cloud-based data management, and personalized cardiac care solutions
Europe Electrophysiology Market Dynamics
Driver
Rising Cardiovascular Disease Prevalence and Advanced Treatment Adoption
- The increasing prevalence of arrhythmias and other cardiovascular disorders, coupled with rising adoption of minimally invasive catheter-based procedures, is a significant driver for the heightened demand for electrophysiology solutions
- For instance, in March 2025, Boston Scientific introduced an advanced mapping catheter for complex arrhythmia treatment, aiming to improve procedural accuracy and reduce patient recovery time
- As patient awareness of cardiac conditions grows, EP devices offer advanced diagnostic and therapeutic capabilities, such as precise ablation and real-time mapping, providing a compelling upgrade over conventional treatments
- Furthermore, healthcare providers’ growing focus on minimally invasive procedures and integrated cardiac care pathways is making electrophysiology solutions essential components of advanced cardiac treatment centers
- The ability to perform outpatient EP procedures, monitor patients remotely, and reduce hospitalization duration are key factors propelling adoption across both established and emerging European healthcare systems
- Government and private healthcare investments in cardiovascular care infrastructure are increasing access to EP devices and supporting market growth in countries such as Germany and France
- Technological advancements, including improved catheter designs, high-resolution mapping systems, and energy-efficient ablation devices, are encouraging clinicians to adopt more advanced EP solution
Restraint/Challenge
High Costs and Regulatory Compliance Hurdles
- Concerns surrounding the high cost of EP devices and stringent regulatory requirements pose significant challenges to broader market penetration. As EP procedures require specialized equipment and training, adoption can be limited in cost-sensitive settings
- For instance, reported reimbursement delays in some European countries have slowed the uptake of advanced mapping and ablation systems in smaller hospitals
- Addressing these challenges through strategic pricing, reimbursement support, and compliance with EU medical device regulations is crucial for building trust among healthcare providers. Companies such as Medtronic emphasize regulatory certifications and safety features in their product offerings to reassure clinical stakeholders
- The relatively high initial investment for advanced ablation catheters and mapping systems compared to conventional cardiac treatments can be a barrier, particularly for smaller clinics or hospitals in emerging markets
- Overcoming these challenges through cost-effective solutions, clinician training programs, and streamlined regulatory approvals will be vital for sustained market growth across Europe
- Limited availability of trained electrophysiologists in certain countries can restrict the adoption of advanced EP procedures despite growing infrastructure
- Variability in healthcare reimbursement policies across European countries may slow the adoption of cutting-edge EP technologies and affect consistent market growth
Europe Electrophysiology Market Scope
The market is segmented on the basis of product, target disease, and end user.
- By Product
On the basis of product, the Europe electrophysiology market is segmented into ablation catheters, laboratory devices, diagnostic catheters, access devices, and other products. The ablation catheters segment dominated the market with the largest market revenue share of 45.7% in 2025, driven by their established efficacy in treating arrhythmias and widespread adoption in hospitals and cardiac centers. Ablation catheters are preferred for their ability to provide minimally invasive treatment with precise targeting of arrhythmic tissue, reducing recovery time and improving patient outcomes. The segment also benefits from continuous innovation in catheter design, energy delivery systems, and navigation technologies, which enhance procedural accuracy and safety. Increasing prevalence of atrial fibrillation and other cardiac arrhythmias across Europe further supports demand. Leading EP device manufacturers are focusing on product enhancements, including AI-assisted mapping compatibility and integration with advanced imaging systems, boosting the segment’s market penetration.
The laboratory devices segment is anticipated to witness the fastest growth rate of 12.8% from 2026 to 2033, driven by rising investments in electrophysiology laboratories and diagnostic infrastructure across emerging European countries. Laboratory devices, including mapping systems, electrophysiology recording equipment, and cardiac simulators, enable clinicians to perform precise diagnostics and pre-procedural planning. Hospitals and academic research centers are increasingly adopting these devices for procedural training, clinical trials, and advanced patient monitoring. Integration with AI and data analytics tools is enhancing the utility of laboratory devices, making them essential for optimizing EP procedures. Growing awareness about cardiovascular diseases and the expansion of EP labs in countries such as Poland and Hungary further fuels adoption.
- By Target Disease
On the basis of target disease, the Europe electrophysiology market is segmented into atrial fibrillation, atrial flutter, Wolff-Parkinson-White syndrome, atrioventricular nodal reentry tachycardia, and others. The atrial fibrillation segment dominated the market with the largest revenue share of 42.3% in 2025, owing to the high prevalence of AF among the aging European population and the critical need for catheter ablation and device-based interventions. Atrial fibrillation management often involves long-term monitoring, which drives demand for advanced EP devices and remote monitoring systems. Hospitals and specialty cardiac centers prioritize solutions that combine accuracy, safety, and minimally invasive procedures for AF patients. The segment benefits from robust reimbursement policies, ongoing clinical studies, and continuous innovations in ablation and mapping technologies. Awareness campaigns and guidelines from European cardiology societies further reinforce the adoption of EP solutions for AF management.
The atrial flutter segment is expected to witness the fastest CAGR of 11.5% from 2026 to 2033, driven by rising diagnosis rates and improved access to electrophysiology procedures. Atrial flutter often coexists with other arrhythmias, making early detection and intervention critical. Advancements in ablation catheter technologies, mapping systems, and procedural protocols are enabling highly effective treatment of atrial flutter. Emerging European countries are increasing the availability of specialized EP labs, promoting outpatient and minimally invasive interventions. Patient preference for quicker recovery and reduced hospitalization is encouraging adoption. Partnerships between device manufacturers and hospitals to train clinicians in atrial flutter ablation techniques are further accelerating market growth.
- By End User
On the basis of end user, the Europe electrophysiology market is segmented into hospitals and ambulatory surgical centers (ASCs). The hospitals segment dominated the market with the largest revenue share of 65.2% in 2025, driven by the availability of advanced infrastructure, trained electrophysiologists, and comprehensive cardiac care facilities. Hospitals provide integrated EP services, including diagnostics, ablation procedures, and post-operative monitoring, making them the primary adopters of electrophysiology devices. The segment benefits from government and private healthcare investments in cardiovascular care, increasing patient access to high-quality EP solutions. Hospitals also lead in adopting advanced mapping systems and AI-assisted devices due to their ability to support complex arrhythmia cases and large patient volumes. The concentration of leading EP device manufacturers in countries such as Germany, France, and the UK further supports the dominance of this segment.
The ASCs segment is expected to witness the fastest growth rate of 13.2% from 2026 to 2033, fueled by the trend toward outpatient minimally invasive procedures and shorter hospital stays. ASCs offer cost-effective and convenient EP treatment options, particularly for ablation procedures and routine diagnostics. Patients increasingly prefer ASCs for faster recovery, reduced risk of hospital-acquired infections, and personalized care. Device manufacturers are tailoring products for use in ASCs, including compact mapping systems and portable ablation catheters. The expansion of ASCs in emerging European markets is creating new opportunities for EP device adoption. Technological improvements and regulatory support are further encouraging the growth of ASCs as key end users of electrophysiology solutions.
Europe Electrophysiology Market Regional Analysis
- Germany dominated the Europe electrophysiology market with the largest revenue share of 38.5% in 2025, characterized by well-established healthcare infrastructure, high adoption of advanced cardiac technologies, and strong presence of leading EP device manufacturers, with substantial growth driven by investments in EP labs and innovations in mapping and ablation systems
- Clinicians and hospitals in Germany prioritize precision, minimally invasive procedures, and advanced mapping technologies, increasing adoption of EP devices such as ablation catheters, diagnostic catheters, and laboratory systems for arrhythmia management
- This widespread adoption is further supported by robust government healthcare investment, favorable reimbursement policies, and a skilled pool of electrophysiologists, establishing Germany as a key hub for EP procedures and advanced cardiac care in Europe
The Germany Electrophysiology Market Insight
The Germany electrophysiology market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced healthcare infrastructure, high awareness of cardiac care, and a strong focus on technological innovation. Germany hosts numerous leading EP device manufacturers and specialized cardiac centers, driving adoption of ablation catheters, diagnostic catheters, and laboratory systems. Government support, favorable reimbursement policies, and a skilled workforce of electrophysiologists further reinforce market growth. Increasing incidence of atrial fibrillation and other arrhythmias is promoting the demand for minimally invasive procedures and AI-assisted EP solutions. Hospitals and specialty cardiac centers are increasingly integrating advanced mapping and ablation systems into patient care pathways.
France Electrophysiology Market Insight
The France electrophysiology market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising cardiovascular disease prevalence, supportive healthcare policies, and high adoption of advanced EP devices. French hospitals and clinics are prioritizing minimally invasive ablation procedures and comprehensive arrhythmia management, increasing demand for diagnostic catheters and mapping systems. Awareness campaigns and clinical guidelines from national cardiology societies are promoting early diagnosis and treatment. Integration of EP devices with remote monitoring platforms and AI-assisted mapping enhances procedural accuracy and patient safety. The strong presence of global EP device manufacturers and investment in research and training is expected to continue stimulating market growth.
Italy Electrophysiology Market Insight
The Italy electrophysiology market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing arrhythmia prevalence and rising investments in cardiac care infrastructure. Hospitals and specialized EP centers are adopting advanced ablation catheters, diagnostic systems, and mapping technologies to improve procedural efficiency and outcomes. Growing awareness of cardiovascular health and patient preference for minimally invasive procedures are driving adoption. Integration with data analytics and AI-assisted mapping tools is becoming more prevalent, enhancing treatment precision. The market growth is further supported by government initiatives promoting advanced healthcare services and reimbursement support for electrophysiology procedures.
Poland Electrophysiology Market Insight
The Poland electrophysiology market is poised to witness the fastest CAGR during the forecast period, driven by rapid expansion of cardiac care facilities and increasing accessibility to advanced EP devices. Hospitals and outpatient centers are adopting ablation catheters, laboratory devices, and mapping systems to meet growing patient demand. Investment in training and infrastructure, coupled with awareness of arrhythmia management, is fueling market growth. AI-assisted mapping and remote monitoring adoption is rising, improving procedural outcomes and patient follow-up. The country’s emphasis on modernizing healthcare infrastructure and expanding minimally invasive cardiac procedures further supports rapid market growth.
Europe Electrophysiology Market Share
The Europe Electrophysiology industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Boston Scientific Corporation (U.S.)
- Biotronik (Germany)
- Medtronic (Ireland)
- GE Healthcare (U.K.)
- Koninklijke Philips N.V. (Netherlands)
- Siemens Healthineers AG (Germany)
- MicroPort Scientific Corporation (China)
- Biosense Webster, Inc. (U.S.)
- AtriCure, Inc. (U.S.)
- CardioFocus, Inc. (U.S.)
- Stereotaxis, Inc. (U.S.)
- Osypka AG (Germany)
- EP Solutions SA (Switzerland)
- LivaNova PLC (U.K.)
- CathVision ApS (Denmark)
- Volta Medical (Netherlands)
- Auris Health, Inc. (U.S.)
- NIHON KOHDEN CORPORATION (Japan)
- Imricor Medical Systems, Inc. (U.S.)
What are the Recent Developments in Europe Electrophysiology Market?
- In July 2025, BIOTRONIK entered a strategic partnership with CardioFocus to expand distribution of the CE‑marked Centauri Pulsed Field Ablation System across 17 European markets, aiming to broaden access to PFA technology that has been used in thousands of patients with efficient lesion creation and low complication rates
- In March 2025, Abbott announced that it received CE Mark approval in Europe for its Volt™ PFA System, a pulsed field ablation platform designed to treat atrial fibrillation with a single‑catheter approach that simplifies workflow and improves procedural effectiveness. This approval enables wider commercial use of Volt PFA across the EU, marking a significant regulatory and clinical milestone for next‑generation EP ablation technology
- In May 2024, Biosense Webster unveiled the CARTO™ 3 System Version 8, an advanced 3D electro‑anatomical mapping system with enhanced machine learning and signal analysis modules (e.g., CARTOSOUND™ FAM and ELEVATE™). This launch represents a major technology upgrade supporting more accurate and efficient arrhythmia mapping for EP procedures
- In April 2024, clinical evidence from the VOLT CE Mark Study was published reporting the safety and effectiveness of a novel balloon‑in‑basket pulsed field ablation system in pulmonary vein isolation (PVI) for atrial fibrillation patients, showing high procedural success and low adverse events a key clinical validation step supporting adoption of PFA technology in Europe
- In February 2024, a new pulsed field ablation (PFA) system received CE Mark in Europe under harmonized nomenclature and definitions for PFA technology, underscoring regulatory advancement and standardization for emerging energy‑based ablation modalities across electrophysiology procedures
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.