Clinical trial imaging plays a pivotal role in healthcare research. Its applications encompass various medical fields, aiding in disease diagnosis, treatment evaluation, and drug development. Advanced imaging techniques such as MRI, CT, and PET offer detailed anatomical and functional insights, supporting precise diagnoses and monitoring therapeutic outcomes. Imaging data helps researchers assess treatment efficacy, track disease progression, and identify adverse effects. This technology ensures accurate data collection, enhancing clinical trials' validity and advancing medical knowledge to benefit patient care and pharmaceutical innovation.
Access full Report @ https://www.databridgemarketresearch.com/reports/north-america-clinical-trial-imaging-market
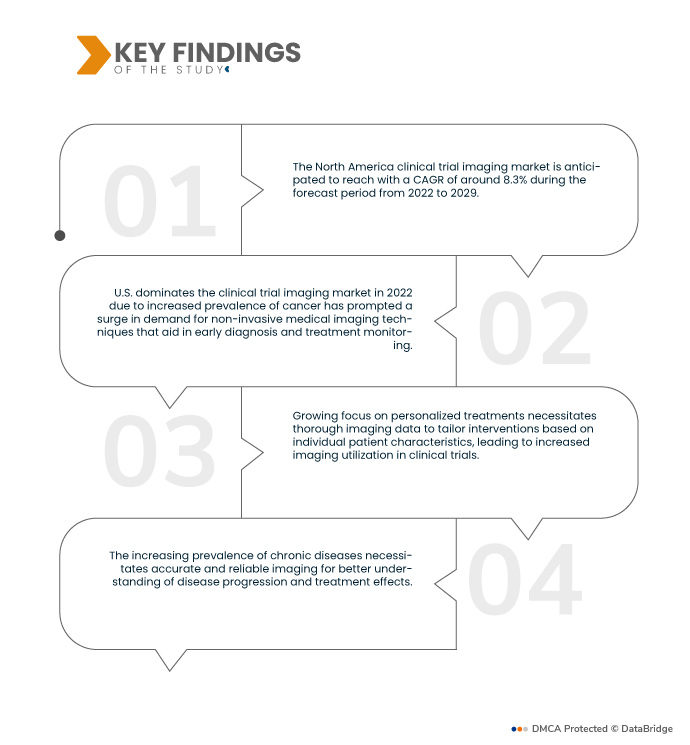
Data Bridge Market Research analyses that the North America Clinical Trial Imaging Market is registering a CAGR of 8.3% during the forecast period of 2022 to 2029. Technological progress in medical imaging, such as MRI, CT, and PET, enables more accurate and detailed visualization of anatomical structures and disease processes, driving demand for imaging services in clinical trials.
Key Findings of the Study
The rise in chronic diseases is expected to drive the market's growth rate
As chronic diseases become more widespread, the development of new treatments becomes imperative. Rigorous testing of these treatments mandates thorough monitoring of disease progression and treatment effectiveness. Comprehensive imaging assessment plays a vital role in this process by providing detailed insights into anatomical changes, treatment responses, and potential adverse effects. This helps researchers and clinicians make informed decisions about the efficacy and safety of novel interventions, ultimately advancing the understanding and management of chronic diseases.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2022 to 2029
|
Base Year
|
2021
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
Segments Covered
|
Product and Services (Services and Software), Modality (Computed Tomography, Magnetic resonance imaging, Echocardiography, Nuclear Medicine, Positron Emission Tomography, X-ray, Ultrasound, Optical Coherence Tomography, and Others), Application (Oncology, Neurology, Endocrinology, Cardiology, Dermatology, Hematology, and Others), End User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Medical Device Manufacturers, Academic and Government Research Institutes and Others), Distributor (Direct Sales and Tender Sales)
|
Countries Covered
|
U.S., Canada, and Mexico
|
Market Players Covered
|
Navitas Life Sciences (U.S.), Resonance Health Analytical Services (Australia), BioTelemetry, a Philips Company (U.S.), ICON plc (Ireland), Quotient Sciences (U.K.), WORLDCARE CLINICAL (U.S.), Clario (U.S.), Paraxel International Corporation (U.S.), Median Technologies (France), Perspectum (U.K.), Calyx (U.S.), WIRB-Copernicus Group (U.S.), Invicro.LLC (U.S.)
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
The North America clinical trial imaging market is segmented on the basis of product and services, modality, application, end user, and distributor.
- On the basis of product and services, the market is segmented into services and software.
- On the basis of modality, the market is segmented into computed tomography, magnetic resonance imaging, echocardiography, nuclear medicine, positron emission tomography, X-ray, ultrasound, Optical Coherence Tomography, and others.
- On the basis of application, the market is segmented into oncology, neurology, cardiology, endocrinology, dermatology, hematology, and others.
- On the basis of end user, the market is segmented into contract research organizations, pharmaceutical and biotechnology companies, medical device manufacturers, academic and government research institutes, and others.
- On the basis of distributor, the market is segmented into direct sales and tender sales.
Major Players
Data Bridge Market Research recognizes the following companies as the major North America clinical trial imaging market players in North America clinical trial imaging market are Navitas Life Sciences (U.S.), Resonance Health Analytical Services (Australia), BioTelemetry, a Philips Company (U.S.), ICON plc (Ireland), Quotient Sciences (U.K.), WORLDCARE CLINICAL (U.S.), Clario (U.S.), Paraxel International Corporation (U.S.)
Market Developments
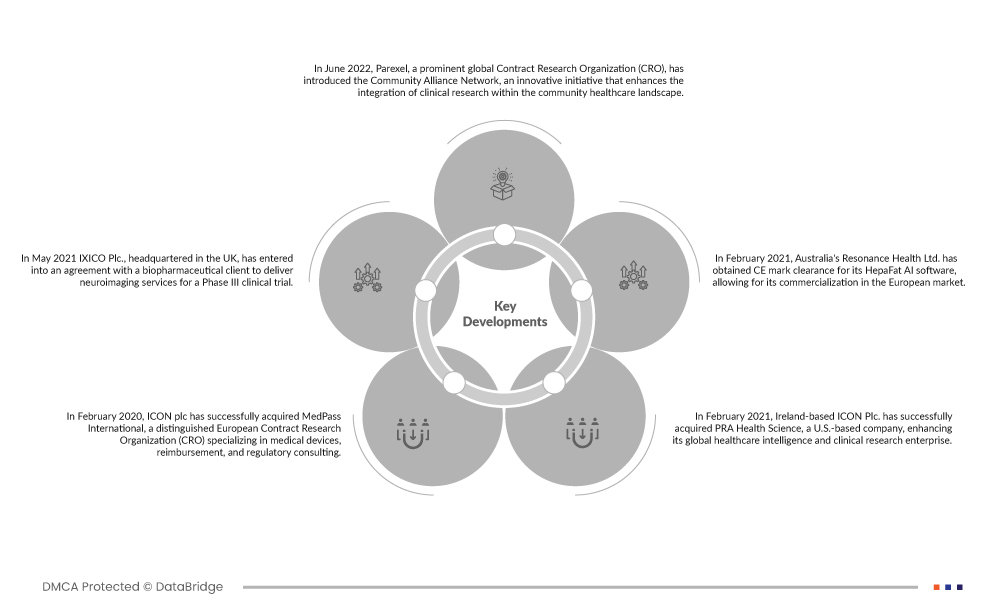
- In June 2022, Parexel, a prominent global Contract Research Organization (CRO), introduced the Community Alliance Network, an innovative initiative that enhances the integration of clinical research within the community healthcare landscape. The program aims to enhance patient care and diversity in clinical trials. Esteemed organizations like CVS Health and Javara have joined as founding members, providing community-based research sites and widening patient access for Parexel's biopharmaceutical partners.
- In February 2020, ICON plc has successfully acquired MedPass International, a distinguished European Contract Research Organization (CRO) specializing in medical devices, reimbursement, and regulatory consulting. This strategic acquisition marks a significant step in enhancing ICON's medical device and diagnostic research offerings across Europe, reinforcing the company's commitment to expanding its footprint and capabilities in this vital sector of the healthcare industry.
- In May 2021 IXICO Plc., headquartered in the UK, has entered into an agreement with a biopharmaceutical client to deliver neuroimaging services for a Phase III clinical trial. This contract entails IXICO providing its specialized expertise in neuroimaging to support the clinical trial's advancement.
- In February 2021, Ireland-based ICON Plc. has successfully acquired PRA Health Science, a U.S.-based company, enhancing its global healthcare intelligence and clinical research enterprise. This strategic move aims to bolster ICON's capabilities and presence in these sectors on a global scale.
- In February 2021, Australia's Resonance Health Ltd. has obtained CE mark clearance for its HepaFat AI software, allowing for its commercialization in the European market.
Regional Analysis
Geographically, the countries covered in the North America clinical trial imaging market report are China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of North America (APAC) in the North America (APAC)
As per Data Bridge Market Research analysis:
U.S. dominates North America in clinical trial imaging market during the forecast period 2022 - 2029
In 2022, U.S. dominates the North America clinical trial imaging market due to technological advancements in clinical trial imaging, such as MRI, CT, and PET, which offer more precise and detailed insights into disease progression. Simultaneously, a rise in research and development activities has intensified the need for comprehensive imaging data to assess treatment effectiveness and drug safety. Collectively, these factors drive the growth of clinical trial imaging, enabling more accurate evaluations of therapies and contributing to medical advancements.
For more detailed information about the clinical trial imaging market report, click here – https://www.databridgemarketresearch.com/reports/north-america-clinical-trial-imaging-market